Impact of TTF-1 Expression on the Prognostic Prediction of Patients with Non-Small Cell Lung Cancer with PD-L1 Expression Levels of 1% to 49%, Treated with Chemotherapy vs. Chemoimmunotherapy: A Multicenter, Retrospective Study
- PMID: 39482992
- PMCID: PMC12016825
- DOI: 10.4143/crt.2024.748
Impact of TTF-1 Expression on the Prognostic Prediction of Patients with Non-Small Cell Lung Cancer with PD-L1 Expression Levels of 1% to 49%, Treated with Chemotherapy vs. Chemoimmunotherapy: A Multicenter, Retrospective Study
Abstract
Purpose: Thyroid transcription factor 1 (TTF-1) expression is a useful predictor of treatment efficacy in advanced non-squamous non-small cell lung cancer (NSCLC). This study aimed to evaluate whether TTF-1 could predict the effectiveness of chemotherapy versus chemoimmunotherapy in patients with non-squamous NSCLC with programmed death ligand-1 (PD-L1) expression between 1% and 49%.
Materials and methods: We conducted a retrospective study of patients with NSCLC who were treated with chemotherapy or chemoimmunotherapy between March 2016 and May 2023. The patients had histologically confirmed NSCLC, stage III-IV or postoperative recurrence, TTF-1 measurements, and PD-L1 expression levels between 1% and 49%. Clinical data were analyzed to evaluate the effect of TTF-1 expression on treatment efficacy.
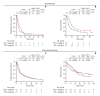
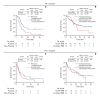
Results: This study included 283 of 624 patients. TTF-1-positive patients showed longer progression-free survival (PFS) and overall survival (OS) (PFS: 6.4 months [95% confidence interval (CI), 5.0 to 9.4] vs. 4.1 months [95% CI, 2.7 to 6.1], p=0.03; OS: 17.9 months [95% CI, 15.2 to 28.1] vs. 9.4 months [95% CI, 6.3 to 17.0], p < 0.01) in the chemotherapy cohorts (n=93). In the chemoimmunotherapy cohort (n=190), there was no significant difference in PFS and OS between TTF-1-positive and -negative groups (PFS: 7.6 months [95% CI, 6.4 to 11.0] vs. 6.0 months [95% CI, 3.6 to 12.6], p=0.59; OS: 25.0 months [95% CI, 18.0 to 49.2] vs. 21.3 months [95% CI, 9.8 to 28.8], p=0.09).
Conclusion: In patients with NSCLC with PD-L1 expression between 1% and 49%, TTF-1 expression was a predictor of chemotherapeutic, but not chemoimmunotherapeutic, efficacy.
Keywords: Chemoimmunotherapy; Chemotherapy; Non-small-cell lung carcinoma; PD-L1; Thyroid transcription factor 1.
Conflict of interest statement
N. Nishioka received personal fees from Chugai Pharmaceutical Co. Ltd., AstraZeneca KK., Eli Lilly Japan KK, and MSD KK, outside the purview of the submitted work. Tadaaki Yamada received research grants from Ono Pharmaceutical, Janssen, AstraZeneca, and Takeda Pharmaceutical and has received speaking honoraria from Eli Lilly and Chugai-Roshe outside the purview of the submitted work. Satoshi Watanabe received grants from Boehringer Ingelheim and Nippon Kayaku and has received honoraria for speakers’ bureaus from Lilly, Novartis Pharma, Chugai Pharma Bristol-Myers, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Nippon Kayaku, Kyowa Kirin, Merck, Takeda Pharmaceutical, Celltrion, and AstraZeneca outside the purview of the submitted work. Hirokazu Taniguchi has received lecture fees from AstraZeneca and Chugai Pharma. Tomoya Fukui received personal fees from AstraZeneca K.K., Boehringer-Ingelheim Japan Inc., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan KK, Nippon Kayaku Co. Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., and Pfizer Japan Inc., outside the scope of the submitted work. Kyoichi Kaira has received speaker honorariums from Ono Pharmaceutical Company, Chugai Pharmaceutical, Bristol-Myers Company, Boehringer Ingelheim, and AstraZeneca, and research grants from AstraZeneca. Asuka Okada has received personal fees from Chugai-Roshe, Kyowa Kirin, MSD KK, AstraZeneca, Takeda Pharmaceutical, Boehringer Ingelheim, Eli Lilly, Japan, Nippon Kayaku, and Bristol-Myers Squibb outside the purview of the submitted work. Hayato Kawachi received personal fees from Bristol-Myers Squibb, Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Taiho Pharmaceutical Co. Ltd., Eli Lilly Japan KK, and MSD KK, outside the purview of the submitted work. Takashi Kijima received personal fees from Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Ono Pharmaceutical Co. Ltd., and MSD KK, outside the purview of the submitted work. Koichi Takayama received research grants from Chugai Pharmaceutical Co. Ltd. and Ono Pharmaceutical and personal fees from AstraZeneca, Chugai Pharmaceutical Co. Ltd., MSD-Merck, Eli Lilly, Boehringer Ingelheim, and Daiichi-Sankyo outside the purview of the submitted work. The other authors declare no potential conflicts of interest.
Figures
References
-
- Pelosi G, Scarpa A, Forest F, Sonzogni A. The impact of immunohistochemistry on the classification of lung tumors. Expert Rev Respir Med. 2016;10:1105–21. - PubMed
-
- Khoor A, Whitsett JA, Stahlman MT, Olson SJ, Cagle PT. Utility of surfactant protein B precursor and thyroid transcription factor 1 in differentiating adenocarcinoma of the lung from malignant mesothelioma. Hum Pathol. 1999;30:695–700. - PubMed
-
- Kaufmann O, Dietel M. Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathology. 2000;36:8–16. - PubMed
-
- Zhang Y, Wang R, Li Y, Pan Y, Hu H, Zhang Y, et al. Negative thyroid transcription factor 1 expression defines an unfavorable subgroup of lung adenocarcinomas. J Thorac Oncol. 2015;10:1444–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

