ZeroCAL: Eliminating Carbon Dioxide Emissions from Limestone's Decomposition to Decarbonize Cement Production
- PMID: 39483210
- PMCID: PMC11523464
- DOI: 10.1021/acssuschemeng.4c03193
ZeroCAL: Eliminating Carbon Dioxide Emissions from Limestone's Decomposition to Decarbonize Cement Production
Abstract
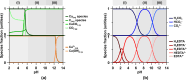
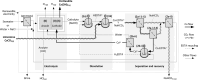
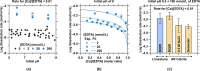
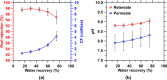
Limestone (calcite, CaCO3) is an abundant and cost-effective source of calcium oxide (CaO) for cement and lime production. However, the thermochemical decomposition of limestone (∼800 °C, 1 bar) to produce lime (CaO) results in substantial carbon dioxide (CO2(g)) emissions and energy use, i.e., ∼1 tonne [t] of CO2 and ∼1.4 MWh per t of CaO produced. Here, we describe a new pathway to use CaCO3 as a Ca source to make hydrated lime (portlandite, Ca(OH)2) at ambient conditions (p, T)-while nearly eliminating process CO2(g) emissions (as low as 1.5 mol. % of the CO2 in the precursor CaCO3, equivalent to 9 kg of CO2(g) per t of Ca(OH)2)-within an aqueous flow-electrolysis/pH-swing process that coproduces hydrogen (H2(g)) and oxygen (O2(g)). Because Ca(OH)2 is a zero-carbon precursor for cement and lime production, this approach represents a significant advancement in the production of zero-carbon cement. The Zero CArbon Lime (ZeroCAL) process includes dissolution, separation/recovery, and electrolysis stages according to the following steps: (Step 1) chelator (e.g., ethylenediaminetetraacetic acid, EDTA)-promoted dissolution of CaCO3 and complexation of Ca2+ under basic (>pH 9) conditions, (Step 2a) Ca enrichment and separation using nanofiltration (NF), which allows separation of the Ca-EDTA complex from the accompanying bicarbonate (HCO3 -) species, (Step 2b) acidity-promoted decomplexation of Ca from EDTA, which allows near-complete chelator recovery and the formation of a Ca-enriched stream, and (Step 3) rapid precipitation of Ca(OH)2 from the Ca-enriched stream using electrolytically produced alkalinity. These reactions can be conducted in a seawater matrix yielding coproducts including hydrochloric acid (HCl) and sodium bicarbonate (NaHCO3), resulting from electrolysis and limestone dissolution, respectively. Careful analysis of the reaction stoichiometries and energy balances indicates that approximately 1.35 t of CaCO3, 1.09 t of water, 0.79 t of sodium chloride (NaCl), and ∼2 MWh of electrical energy are required to produce 1 t of Ca(OH)2, with significant opportunity for process intensification. This approach has major implications for decarbonizing cement production within a paradigm that emphasizes the use of existing cement plants and electrification of industrial operations, while also creating approaches for alkalinity production that enable cost-effective and scalable CO2 mineralization via Ca(OH)2 carbonation.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











References
-
- De Brito J.; Kurda R. The Past and Future of Sustainable Concrete: A Critical Review and New Strategies on Cement-Based Materials. Journal of Cleaner Production 2021, 281, 123558. 10.1016/j.jclepro.2020.123558. - DOI
-
- Barcelo L.; Kline J.; Walenta G.; Gartner E. Cement and Carbon Emissions. Mater. Struct 2014, 47 (6), 1055–1065. 10.1617/s11527-013-0114-5. - DOI
-
- Strunge T.; Naims H.; Ostovari H.; Olfe-Kräutlein B. Priorities for Supporting Emission Reduction Technologies in the Cement Sector - A Multi-Criteria Decision Analysis of CO2 mineralisation. Journal of Cleaner Production 2022, 340, 130712. 10.1016/j.jclepro.2022.130712. - DOI
-
- Bustillo Revuelta M. L.Construction Materials; Springer Textbooks in Earth Sciences, Geography and Environment; Springer International Publishing: Cham, Switzerland, 2021; pp 167–193; 10.1007/978-3-030-65207-4_7. - DOI
-
- FitzGerald D.; Bourgault G.; Vadenbo C.; Sonderegger T.; Symeonidis A.; Fazio S.; Mutel C.; Müller J.; Dellenbach D.; Stoikou N.; Baumann D.; Clementi M.; Ioannou I.; Cirone F.; Superti V.; Beckert P.; Treichel A.; Kaarlela O.; Kunde S.; Valsasina L.; Moreno Ruiz E.. Documentation of Changes Implemented in the Ecoinvent Database v3.10, 2023; https://19913970.fs1.hubspotusercontent-na1.net/hubfs/19913970/Knowledge....
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
