Square-Planar Nickel Bis(phosphinopyridyl) Complexes for Long-Lived Photocatalytic Hydrogen Evolution
- PMID: 39483239
- PMCID: PMC11522921
- DOI: 10.1021/jacsau.4c00714
Square-Planar Nickel Bis(phosphinopyridyl) Complexes for Long-Lived Photocatalytic Hydrogen Evolution
Abstract
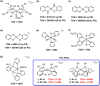
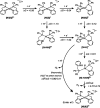
Phosphinopyridyl ligands are used to synthesize a class of Ni(II) bis(chelate) complexes, which have been comprehensively characterized in both solid and solution phases. The structures display a square-planar configuration within the primary coordination sphere, with axially positioned labile binding sites. Their electrochemical data reveal two redox couples during the reduction process, suggesting the possibility of accessing two-electron reduction states. Significantly, these complexes serve as robust catalysts for homogeneous photocatalytic H2 evolution. In a system utilizing an organic photosensitizer and a sacrificial electron donor, an optimal turnover number of 27,100 is achieved in an alcohol-containing aqueous solution. A series of photophysical and electrochemical measurements were conducted to elucidate the reaction mechanism of photocatalytic hydrogen generation. Density function theory calculations propose a catalytic pathway involving two successive one-electron reduction steps, followed by two proton discharges. The sustained photocatalytic activity of these complexes stems from their distinct ligand system, which includes phosphine and pyridine donors that aid in stabilizing the low oxidation states of the Ni center.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Bullock R. M.; Chen J. G.; Gagliardi L.; Chirik P. J.; Farha O. K.; Hendon C. H.; Jones C. W.; Keith J. A.; Klosin J.; Minteer S. D.; Morris R. H.; Radosevich A. T.; Rauchfuss T. B.; Strotman N. A.; Vojvodic A.; Ward T. R.; Yang J. Y.; Surendranath Y. Using nature’s blueprint to expand catalysis with Earth-abundant metals. Science 2020, 369 (6505), eabc318310.1126/science.abc3183. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
