Telomere maintenance and the DNA damage response: a paradoxical alliance
- PMID: 39483338
- PMCID: PMC11524846
- DOI: 10.3389/fcell.2024.1472906
Telomere maintenance and the DNA damage response: a paradoxical alliance
Abstract
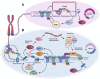
Telomeres are the protective caps at the ends of linear chromosomes of eukaryotic organisms. Telomere binding proteins, including the six components of the complex known as shelterin, mediate the protective function of telomeres. They do this by suppressing many arms of the canonical DNA damage response, thereby preventing inappropriate fusion, resection and recombination of telomeres. One way this is achieved is by facilitation of DNA replication through telomeres, thus protecting against a "replication stress" response and activation of the master kinase ATR. On the other hand, DNA damage responses, including replication stress and ATR, serve a positive role at telomeres, acting as a trigger for recruitment of the telomere-elongating enzyme telomerase to counteract telomere loss. We postulate that repression of telomeric replication stress is a shared mechanism of control of telomerase recruitment and telomere length, common to several core telomere binding proteins including TRF1, POT1 and CTC1. The mechanisms by which replication stress and ATR cause recruitment of telomerase are not fully elucidated, but involve formation of nuclear actin filaments that serve as anchors for stressed telomeres. Perturbed control of telomeric replication stress by mutations in core telomere binding proteins can therefore cause the deregulation of telomere length control characteristic of diseases such as cancer and telomere biology disorders.
Keywords: DNA damage response; nuclear actin; replication stress; shelterin; telomerase; telomere maintenance; telomere replication.
Copyright © 2024 Harman and Bryan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Arnoult N., Schluth-Bolard C., Letessier A., Drascovic I., Bouarich-Bourimi R., Campisi J., et al. (2010). Replication timing of human telomeres is chromosome arm–specific, influenced by subtelomeric structures and connected to nuclear localization. PLoS Genet. 6 (4), e1000920. 10.1371/journal.pgen.1000920 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

