State of the ART (antiretroviral therapy): Long-acting HIV-1 therapeutics
- PMID: 39483451
- PMCID: PMC11514626
- DOI: 10.35772/ghm.2024.01049
State of the ART (antiretroviral therapy): Long-acting HIV-1 therapeutics
Abstract
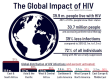
Human immunodeficiency virus (HIV) impacts millions of individuals worldwide, and well over 2/3 of those living with HIV are accessing antiviral therapies that are successfully repressing viral replication. Most often, HIV treatments and prevention are administered in the form of daily pills as combinations of multiple drugs. An emergent and effective strategy for suppressing viral replication is the application of long-acting antiretroviral therapy (LAART), or antivirals that require less-frequent, non-daily doses. Thus far, the repertoire of LAARTs includes the widely used antiviral classes of non-nucleoside reverse transcriptase inhibitors (NNRTIs) and integrase strand transfer inhibitors (INSTIs) and has recently expanded to include a capsid-targeting antiviral. Possible future additions are nucleoside reverse transcriptase inhibitors (NRTIs) and nucleoside reverse transcriptase translocation inhibitors (NRTTIs). Here, we discuss the different strategies of using long-acting compounds to treat or prevent HIV-1 infection by targeting reverse transcriptase, integrase, and capsid.
Keywords: acquired immunodeficiency syndrome (AIDS); antiretroviral therapy (ART); human immunodeficiency virus (HIV); long-acting formulations; pre-exposure prophylaxis (PrEP).
2024, National Center for Global Health and Medicine.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- UNAIDS. Global HIV & AIDS statistics - Fact Sheet Geneva, Switzerland 2024. https://www.unaids.org/en/resources/fact-sheet (accessed June 28, 2024).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

