In silico and pharmacological evaluation of GPR65 as a cancer immunotherapy target regulating T-cell functions
- PMID: 39483470
- PMCID: PMC11525786
- DOI: 10.3389/fimmu.2024.1483258
In silico and pharmacological evaluation of GPR65 as a cancer immunotherapy target regulating T-cell functions
Abstract
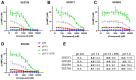
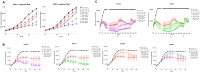
The success of cancer immunotherapies such as immune checkpoint inhibitors, CAR T-cells and immune cell engagers have provided clinicians with tools to bypass some of the limitations of cancer immunity. However, numerous tumour factors curtail the immune response against cancer and limit the efficiency of immuno-oncology (IO) therapies. Acidification of the extra-cellular tumour environment consecutive to aberrant cancer cell metabolism is a well-known promoter of oncogenic processes that also acts as an immune regulator. Yet, the suppressive mechanisms of low extra-cellular pH on anti-cancer immunity remain poorly understood. Recent reports have suggested that GPR65, a Gαs-coupled proton-sensing GPCR broadly expressed in the immune system, may act as an immune suppressant detrimental to anti-tumour immunity. So far, the immuno-regulatory properties of GPR65 in acidic milieux have mostly been documented in macrophages and myeloid cells. Our computational evaluation of GPR65's transcriptomic expression profile and potential as an IO target using public datasets prompted us to further investigate its functions in human T-cells. To this end, we identified and validated GPR65 small molecule inhibitors active in in vitro cellular assays and we showed that GPR65 inhibition promoted the killing capacity of antigen-specific human T-cells. Our results broaden the scope of GPR65 as an IO target by suggesting that its inhibition may enhance T-cell anti-tumour activity and provide useful pharmacological tools to further investigate the therapeutic potential of GPR65 inhibition.
Keywords: GPCRs; Gpr65; T-cells; TDAG8; acidosis; immunosuppression; immunotherapy; solid tumours.
Copyright © 2024 Li, Melchiore, Kantari-Mimoun, Mouton, Knockaert, Philippon, Chanrion, Bourgeois, Lefebvre, Elhmouzi-Younes, Blanc, Ramon Olayo and Laugel.
Conflict of interest statement
All authors are or were employees of Laboratoires Servier, who sponsored the research. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

