Mesenchymal stem cells therapy for chronic ischemic stroke-a systematic review
- PMID: 39483715
- PMCID: PMC11524678
- DOI: 10.2478/abm-2024-0027
Mesenchymal stem cells therapy for chronic ischemic stroke-a systematic review
Abstract
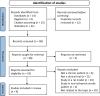
Stroke represents a significant global health issue, primarily in the form of ischemic stroke. Despite the availability of therapeutic interventions, the recovery from chronic stroke, occurring 3 months post-initial stroke, poses substantial challenges. A promising avenue for post-acute stroke patients is mesenchymal stem cells (MSCs) therapy, which is derived from various sources and is globally recognized as the most utilized and extensively studied stem cell therapy. This systematic review, adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, aims to synthesize evidence regarding the impact of MSCs therapy on patients with chronic ischemic stroke. Employing an advanced search strategy across databases such as PubMed, PubMed Central, Google Scholar, the Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrial.gov, a total of 70 studies were identified, with 4studies meeting the inclusion criteria. Although positive outcomes were observed in terms of efficacy and safety, certain limitations, such as small sample sizes, study heterogeneity, and the absence of placebo groups, undermine the overall strength of the evidence. It is crucial to address these limitations in future research, highlighting the importance of larger sample sizes, standardized methodologies, and comparative trials to improve the assessment of MSCs' efficacy and safety. Moving forward, key priorities include exploring underlying mechanisms, determining optimal administration modes and dosages, and conducting comparative trials. By addressing these aspects, we can propel MSCs therapies toward greater efficacy, safety, and applicability across diverse patient populations.
Keywords: chronic; ischemic stroke; mesenchymal stem cells; stroke; treatment.
© 2024 Mohammad Kurniawan et al., published by Sciendo.
Figures
References
-
- Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–11. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
