The Effect of Doxapram on Proprioceptive Neurons: Invertebrate Model
- PMID: 39483770
- PMCID: PMC11523696
- DOI: 10.3390/neurosci3040041
The Effect of Doxapram on Proprioceptive Neurons: Invertebrate Model
Abstract
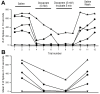
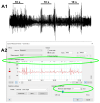
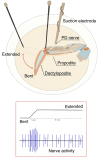
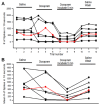
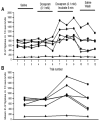
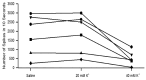
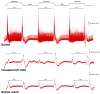
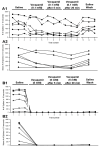
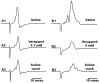
The resting membrane potential enables neurons to rapidly initiate and conduct electrical signals. K2p channels are key in maintaining this membrane potential and electrical excitability. They direct the resting membrane potential toward the K+ equilibrium potential. Doxapram is a known blocker for a subset of K2p channels that are pH sensitive. We assessed the effects of 0.1 and 5 mM doxapram on the neural activity within the propodite-dactylopodite (PD) proprioceptive sensory organ in the walking legs of blue crabs (Callinectes sapidus). Results indicate that 0.1 mM doxapram enhances excitation, while the higher concentration 5 mM may over-excite the neurons and promote a sustained absolute refractory period until the compound is removed. The effect of 5 mM doxapram mimics the effect of 40 mM K+ exposure. Verapamil, another known K2p channel blocker as well as an L-type Ca2+ channel blocker, reduces neural activity at both 0.1 and 5 mM. Verapamil may block stretch activated channels in sensory endings, in addition to reducing the amplitude of the compound action potential with whole nerve preparations. These findings are notable as they demonstrate that doxapram has acute effects on neurons of crustaceans, suggesting a targeted K2p channel. The actions of verapamil are complex due to the potential of affecting multiple ion channels in this preparation. Crustacean neurons can aid in understanding the mechanisms of action of various pharmacological agents as more information is gained.
Keywords: K2p channels; crab; proprioception; sensory.
© 2022 by the authors.
Conflict of interest statement
Conflicts of InterestThe authors declare that they have no conflict of interest.
Figures






References
-
- Plant L.D., Goldstein S.A.N. Two-Pore Domain Potassium Channels. In: Zheng J., Trudeau M.C., editors. Handbook of Ion Channels. 1st ed. CRC Press; Boca Raton, FL, USA: 2015.
LinkOut - more resources
Full Text Sources
Miscellaneous

