This is a preprint.
Phase I First-in-Human Dose Escalation Study of the oral Casein Kinase 1α and Cyclin Dependent Kinase 7/9 inhibitor BTX-A51 in advanced MDS and AML
- PMID: 39483885
- PMCID: PMC11527261
- DOI: 10.21203/rs.3.rs-4954060/v1
Phase I First-in-Human Dose Escalation Study of the oral Casein Kinase 1α and Cyclin Dependent Kinase 7/9 inhibitor BTX-A51 in advanced MDS and AML
Update in
-
Phase I first-in-human dose escalation study of the oral casein kinase 1α and cyclin dependent kinase 7/9 inhibitor BTX A51 in advanced MDS and AML.J Hematol Oncol. 2025 Jul 15;18(1):73. doi: 10.1186/s13045-025-01724-z. J Hematol Oncol. 2025. PMID: 40665325 Free PMC article. Clinical Trial.
Abstract
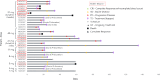
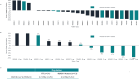
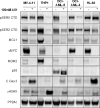
BTX-A51, a first-in-class oral small molecule inhibitor of casein kinase 1α (CK1α) and cyclin dependent kinase (CDK) 7 and 9, induces apoptosis of leukemic cells by activating p53 and inhibiting expression of Mcl1. Here, we report on the results of the phase 1 clinical trial of BTX-A51 in patients with relapsed or refractory AML and MDS. Thirty-one patients were enrolled into 8 dose-escalation cohorts at BTX-A51 doses ranging from 1mg to 42mg dosed three days/week for 21 or 28 days out a 28-day cycle. The recommended phase 2 dose was 21mg dosed three days/week for 4 weeks of a 28-day cycle. BTX-A51 increased expression of p53 and reduced expression of MCL1 and RNA polymerase II phosphorylation on pre- and post-treatment immunocytochemistry studies. Overall, 3 patients (10%) experienced complete remission with incomplete count recovery (CRi). All 3 responding patients had RUNX1 mutations and the CR/CRi rate for RUNX1-mutated patients receiving BTX-A51 at efficacious doses (11mg or higher) was 30%. Ex-vivo studies confirmed higher efficacy of BTX-A51 on RUNX1-mutated myeloblasts and demonstrate synergy with azacitidine and venetoclax. Although the overall efficacy was modest, this study lays the groundwork for future studies with improved patient selection and combination approaches.
Figures

References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. 2022;72(1):7–33. - PubMed
-
- Bernard E, Tuechler H, Greenberg Peter L, Hasserjian Robert P, Arango Ossa Juan E, Nannya Y, et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evidence. 2022;1(7):EVIDoa2200008. - PubMed
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. New England Journal of Medicine. 2015;373(12):1136–52. - PubMed
-
- Wattad M, Weber D, Döhner K, Krauter J, Gaidzik VI, Paschka P, et al. Impact of salvage regimens on response and overall survival in acute myeloid leukemia with induction failure. Leukemia. 2017;31(6):1306–13. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

