Characterization of GABAergic marker expression in prefrontal cortex in dexamethasone induced depression/anxiety model
- PMID: 39483976
- PMCID: PMC11524930
- DOI: 10.3389/fendo.2024.1433026
Characterization of GABAergic marker expression in prefrontal cortex in dexamethasone induced depression/anxiety model
Abstract
Background: The pivotal responsibility of GABAergic interneurons is inhibitory neurotransmission; in this way, their significance lies in regulating the maintenance of excitation/inhibition (E/I) balance in cortical circuits. An abundance of glucocorticoids (GCs) exposure results in a disorder of GABAergic interneurons in the prefrontal cortex (PFC); the relationship between this status and an enhanced vulnerability to neuropsychiatric ailments, like depression and anxiety, has been identified, but this connection is still poorly understood because systematic and comprehensive research is lacking. Here, we aim to investigate the impact of dexamethasone (DEX, a GC receptor agonist) on GABAergic interneurons in the PFC of eight-week-old adult male mice.
Methods: A double-blind study was conducted where thirty-two mice were treated subcutaneously either saline or DEX (0.2 mg/10 ml per kg of body weight) dissolved in saline daily for 21 days. Weight measurements were taken at five-day intervals to assess the emotional changes in mice as well as the response to DEX treatment. Following the 21-day regimen of DEX injections, mice underwent examinations for depression/anxiety-like behaviours and GABAergic marker expression in PFC.
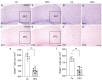
Results: In a depression/anxiety model generated by chronic DEX treatment, we found that our DEX procedure did trigger depression/anxiety-like behaviors in mice. Furthermore, DEX treatment reduced the expression levels of a GABA-synthesizing enzyme (GAD67), Reelin, calcium-binding proteins (parvalbumin and calretinin) and neuropeptides co-expressed in GABAergic neurons (somatostatin, neuropeptide Y and vasoactive intestinal peptide) in the PFC were reduced after 21 days of DEX treatment; these reductions were accompanied by decreases in brain size and cerebral cortex thickness.
Conclusion: Our results indicate that a reduction in the number of GABAergic interneurons may result in deficiencies in cortical inhibitory neurotransmission, potentially causing an E/I imbalance in the PFC; this insight suggests a potential breakthrough strategy for the treatment of depression and anxiety.
Keywords: GABAergic interneurons; GAD67; chronic dexamethasone stress; chronic restraint stress; depression and anxiety; prefrontal cortex; reelin.
Copyright © 2024 Hu, Qiu, Fan, Wang, Liu, Liu, Liu, Shen, Zheng, Liu, Jia, Wang and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

