Cancer metabolic reprogramming and precision medicine-current perspective
- PMID: 39484162
- PMCID: PMC11524845
- DOI: 10.3389/fphar.2024.1450441
Cancer metabolic reprogramming and precision medicine-current perspective
Abstract
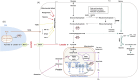
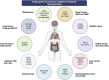
Despite the advanced technologies and global attention on cancer treatment strategies, cancer continues to claim lives and adversely affects socio-economic development. Although combination therapies were anticipated to eradicate this disease, the resilient and restorative nature of cancers allows them to proliferate at the expense of host immune cells energetically. This proliferation is driven by metabolic profiles specific to the cancer type and the patient. An emerging field is exploring the metabolic reprogramming (MR) of cancers to predict effective treatments. This mini-review discusses the recent advancements in cancer MR that have contributed to predictive, preventive, and precision medicine. Current perspectives on the mechanisms of various cancer types and prospects for MR and personalized cancer medicine are essential for optimizing metabolic outputs necessary for personalized treatments.
Keywords: immune cell; mechanism; metabolic reprogramming; precision medicine; treatment strategies.
Copyright © 2024 Gao, Yang, Zhang, Bajinka and Yuan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abou Khouzam R., Sharda M., Rao S. P., Kyerewah-Kersi S. M., Zeinelabdin N. A., Mahmood A. S., et al. (2023). Chronic hypoxia is associated with transcriptomic reprogramming and increased genomic instability in cancer cells. Front. Cell Dev. Biol. 11, 1095419. 10.3389/fcell.2023.1095419 - DOI - PMC - PubMed
-
- Alistar A., Morris B. B., Desnoyer R., Klepin H. D., Hosseinzadeh K., Clark C., et al. (2017). Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 18 (6), 770–778. 10.1016/S1470-2045(17)30314-5 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

