Fluconazole worsened lung inflammation, partly through lung microbiome dysbiosis in mice with ovalbumin-induced asthma
- PMID: 39484217
- PMCID: PMC11526796
- DOI: 10.7717/peerj.18421
Fluconazole worsened lung inflammation, partly through lung microbiome dysbiosis in mice with ovalbumin-induced asthma
Abstract
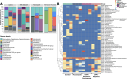
Innate immunity in asthma may be influenced by alterations in lung microbiota, potentially affecting disease severity. This study investigates the differences in lung inflammation and microbiome between asthma-ovalbumin (OVA) administered with and without fluconazole treatment in C57BL/6 mice. Additionally, the role of inflammation was examined in an in vitro study using a pulmonary cell line. At 30 days post-OVA administration, allergic asthma mice exhibited increased levels of IgE and IL-4 in serum and lung tissue, higher pathological scores, and elevated eosinophils in bronchoalveolar lavage fluid (BALF) compared to control mice. Asthma inflammation was characterized by elevated serum IL-6, increased lung cytokines (TNF-α, IL-6, IL-10), and higher fungal abundance confirmed by polymerase chain reaction (PCR). Fluconazole-treated asthma mice displayed higher levels of cytokines in serum and lung tissue (TNF-α and IL-6), increased pathological scores, and a higher number of mononuclear cells in BALF, with undetectable fungal levels compared to untreated mice. Lung microbiome analysis revealed similarities between control and asthma mice; however, fluconazole-treated asthma mice exhibited higher Bacteroidota levels, lower Firmicutes, and reduced bacterial abundance. Pro-inflammatory cytokine production was increased in supernatants of the pulmonary cell line (NCI-H292) after co-stimulation with LPS and beta-glucan (BG) compared to LPS alone. Fluconazole treatment in OVA-induced asthma mice exacerbated inflammation, partially due to fungi and Gram-negative bacteria, as demonstrated by LPS+BG-activated pulmonary cells. Therefore, fluconazole should be reserved for treating fungal asthma rather than asthma caused by other etiologies.
Keywords: Asthma; Fungi; Microbiome; Ovalbumin.
© 2024 Worasilchai et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

