This is a preprint.
Sputum and tongue swab molecular testing for the in-home diagnosis of tuberculosis in unselected household contacts: a cost and cost-effectiveness analysis
- PMID: 39484233
- PMCID: PMC11527052
- DOI: 10.1101/2024.10.18.24315746
Sputum and tongue swab molecular testing for the in-home diagnosis of tuberculosis in unselected household contacts: a cost and cost-effectiveness analysis
Update in
-
Sputum and tongue swab molecular testing for the in-home diagnosis of tuberculosis in unselected household contacts: a cost and cost-effectiveness analysis.Clin Infect Dis. 2025 Jul 24:ciaf389. doi: 10.1093/cid/ciaf389. Online ahead of print. Clin Infect Dis. 2025. PMID: 40705837
Abstract
Background: Delayed and missed diagnosis are a persistent barrier to tuberculosis control, partly driven by limitations associated with sputum collection and an unmet need for decentralized testing. Household contact investigation with point-of-care testing of non-invasive specimens like tongue swabs are hitherto undescribed and may be a cost-effective solution to enable community-based active case finding.
Methods: In-home, molecular point-of-care testing was conducted using sputum and tongue specimens collected from all household contacts of confirmed tuberculosis cases. A health economic assessment was executed to estimate and compare the cost and cost-effectiveness of different in-home, point-of-care testing strategies. Incremental cost effectiveness ratios of strategies utilizing different combination testing algorithms using sputum and/or tongue swab specimens were compared.
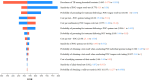
Findings: The total implementation cost of delivering the standard of care for a 2-year period was $84 962. Strategies integrating in-home point-of-care testing ranged between $87 844 - $93 969. The cost-per-test for in-home, POC testing of sputum was the highest at $20·08 per test. Two strategies, Point-of-Care Sputum Testing and Point-of-Care Combined Sputum and Individual Tongue Swab Testing were the most cost-effective with ICERs of $543·74 and $547·29 respectively, both below a $2,760 willingness-to-pay threshold.
Interpretation: An in-home, point-of-care molecular testing strategy utilizing combination testing of tongue swabs and sputum specimens would incur an additional 10.6% program cost, compared to SOC, over a 2-year period. The increased sample yield from tongue swabs combined with immediate result notification following, in-home POC testing would increase the number of new TB cases detected and linked to care by more than 800%.
Conflict of interest statement
Conflicts of Interest: None
Figures

References
-
- Global tuberculosis report 2023. Geneva: World Health Organization; 2023. License: CC BY-NC-SA 3.0 IGO.
-
- Sachdeva KS, Kumar N. Closing the gaps in tuberculosis detection—considerations for policy makers. The Lancet Global Health. 2023. Feb 1;11(2):e185–6. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
