This is a preprint.
Comparison of immunogenicity of adjuvanted and high dose influenza vaccination in long-term care facility residents
- PMID: 39484240
- PMCID: PMC11527040
- DOI: 10.1101/2024.10.14.24315459
Comparison of immunogenicity of adjuvanted and high dose influenza vaccination in long-term care facility residents
Abstract
Background: The CDC recommends the more immunogenic adjuvanted and high-dose flu vaccines over standard-dose, non-adjuvanted vaccines for individuals above 65 years old. The current study compares adjuvanted trivalent inactivated flu vaccine (aTIV, FLUAD) versus high-dose flu vaccine (HD-IIV3, FLUZONE HD) to determine if they met non-inferiority standards for older long-term care facility (LTCF) residents.
Methods: We collected blood from long-term care facility residents participating in a randomized 1:1 active control trial comparing MF59C.1 adjuvanted trivalent inactivated flu vaccine, aTIV versus HD-IIV3 over the course of two flu seasons, 2018-2019 and 2019-2020 (Trial, NCT03694808). We assessed humoral immunity at set time points via hemagglutinin inhibition assays (HAI) and anti-neuraminidase (enzyme-linked lectin assays (ELLA)). The recombinant influenza vaccine (RIV, Flublok) was assessed similarly in year two for a small number of participants who were carried over from year 1 (n=32).
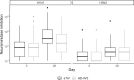
Results: We enrolled 387 volunteers and administered either aTIV (n=194), HD-IIV3 (n=193) over the course of the 2018-2019 and 2019-2020 flu seasons. Among those enrolled and randomized in year one, a subset were administered RIV and studied in year two (n = 32). At 28 days post-vaccination, aTIV exhibited non-inferiority to HD-IIV3 for HAI for both H1N1 and H3N2 strains (GMT ratios (95% CI) for HD-IIV3/aTIV of 1.03(0.76, 1.4) and 1.04(0.73, 1.48), respectively; both 95% CI upper bounds < 1.5 to meet non-inferiority criteria) but not for Influenza B (GMT ratio (95% CI) = 1.21 (0.91, 1.61)). Non-inferiority criteria for HAI seroconversion were not met for any of the three strains. Applying the same non-inferiority criteria to neuraminidase inhibition (NI), both day 28 titer and seroconversion in aTIV were non-inferior to HD-IIV3 for H1N1 and H3N2 strains.
Conclusions: Both aTIV and HD-IIV3 elicited similar immune responses with robust antibody responses. For the primary outcome, aTIV is non-inferior to HD-IIV3 for HAI titer of H1N1 and H3N2 but failed to meet non-inferiority criteria for Influenza B and seroconversion for all assessed strains. For the secondary outcome, aTIV was non-inferior to HD-IIV3 for both titer and seroconversion of anti-neuraminidase for both H1N1 and H3N2.
Keywords: Adjuvanted vaccine; Aged; High-Dose vaccine; Immunogenicity; Influenza vaccine; Long-term care.
Conflict of interest statement
Conflicts of Interest. Potential conflicts of interest. S. G. and D. H. C. are recipients of investigator-initiated grants to their universities from Pfizer to study pneumococcal vaccines, Sanofi Pasteur and Seqirus to study influenza vaccines, and Moderna to study respiratory infection. S. G. is recipient of investigator-initiated grant to the university from Genentech on influenza antivirals; reports consulting for Seqirus, Sanofi, Merck, Vaxart, Novavax, Moderna, and Janssen; has served on the speaker bureaus for Seqirus and Sanofi; reports personal fees from Pfizer; and reports data and safety monitoring board fees from Longevoron and SciClone.
Figures

References
-
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014;371(7):635–645. - PubMed
-
- Gravenstein S, Davidson HE, Taljaard M, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med 2017;5(9):738–746. - PubMed
-
- Gravenstein S, McConeghy KW, Saade E, et al. Adjuvanted influenza vaccine and influenza outbreaks in U.S. nursing homes: Results from a pragmatic cluster-randomized clinical trial. Clin Infect Dis 2021. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
