This is a preprint.
Safety and implementation of a phase 1 randomized GLA-SE-adjuvanted CH505TF gp120 HIV vaccine trial in newborns
- PMID: 39484284
- PMCID: PMC11527060
- DOI: 10.1101/2024.10.15.24315548
Safety and implementation of a phase 1 randomized GLA-SE-adjuvanted CH505TF gp120 HIV vaccine trial in newborns
Update in
-
Safety and implementation of phase I randomized GLA-SE-adjuvanted CH505TF gp120 HIV vaccine trial in newborns.J Clin Invest. 2025 Apr 3;135(11):e186927. doi: 10.1172/JCI186927. eCollection 2025 Jun 2. J Clin Invest. 2025. PMID: 40178906 Free PMC article. Clinical Trial.
Abstract
Background: The neonatal immune system is uniquely poised to generate broadly neutralizing antibodies (bnAbs) and thus infants are ideal for evaluating HIV vaccine candidates. We present the design and safety of a novel glucopyranosyl lipid A (GLA)-stable emulsion (SE) adjuvant admixed with a first-in-infant CH505 transmitter-founder (CH505TF) gp120 immunogen designed to induce precursors for bnAbs against HIV.
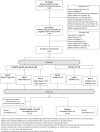
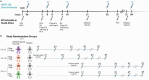
Methods: HVTN 135 is a phase I randomized, placebo-controlled trial of CH505TF+GLA-SE or placebo. Healthy infants in South Africa aged ≤5 days, born to mothers living with HIV but HIV nucleic acid negative at birth were randomized to five doses of CH505TF + GLA-SE or placebo at birth and 8, 16, 32, and 54 weeks.
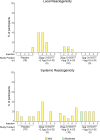
Results: 38 infants (median age = 4 days; interquartile range 4, 4.75 days) were enrolled November 2020 to January 2022. Among 28 (10) infants assigned to receive CH505TF + GLA-SE (placebo), most (32/38) completed the 5-dose immunization series and follow-up (35/38). Solicited local and systemic reactions were more frequent in vaccine (8, 28.6% local; 16, 57.1% systemic) vs. placebo recipients (1, 10% local, p = 0.25; 4, 40.0% systemic, p = 0.38). All events were Grade 1 except two Grade 2 events (pain, lethargy). Serious vaccine-related adverse events were not recorded.
Conclusions: This study illustrates the feasibility of conducting trials of novel adjuvanted HIV vaccines in HIV-exposed infants receiving standard infant vaccinations. The safety profile of the CH505TF + GLA-SE vaccine was reassuring.
Trial registration: ClinicalTrials.gov NCT04607408.
Funding: National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH).
Conflict of interest statement
Declaration of interests OL is a named inventor on patents relating to vaccine adjuvants and to human in vitro systems that model the safety and immunogenicity of adjuvants, vaccines and immunomodulators. He serves as a consultant to GSK and Hillevax and is a co-founder of Ovax, Inc. All other authors declare no conflict of interest.
Figures
References
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
