This is a preprint.
The language network ages well: Preserved selectivity, lateralization, and within-network functional synchronization in older brains
- PMID: 39484368
- PMCID: PMC11527140
- DOI: 10.1101/2024.10.23.619954
The language network ages well: Preserved selectivity, lateralization, and within-network functional synchronization in older brains
Abstract
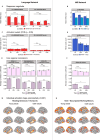
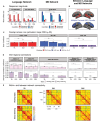
Healthy aging is associated with structural and functional brain changes. However, cognitive abilities differ from one another in how they change with age: whereas executive functions, like working memory, show age-related decline, aspects of linguistic processing remain relatively preserved (Hartshorne et al., 2015). This heterogeneity of the cognitive-behavioral landscape in aging predicts differences among brain networks in whether and how they should change with age. To evaluate this prediction, we used individual-subject fMRI analyses ('precision fMRI') to examine the language-selective network (Fedorenko et al., 2024) and the Multiple Demand (MD) network, which supports executive functions (Duncan et al., 2020), in older adults (n=77) relative to young controls (n=470). In line with past claims, relative to young adults, the MD network of older adults shows weaker and less spatially extensive activations during an executive function task and reduced within-network functional synchronization. However, in stark contrast to the MD network, we find remarkable preservation of the language network in older adults. Their language network responds to language as strongly and selectively as in younger adults, and is similarly lateralized and internally synchronized. In other words, the language network of older adults looks indistinguishable from that of younger adults. Our findings align with behavioral preservation of language skills in aging and suggest that some networks remain young-like, at least on standard measures of function and connectivity.
Keywords: aging; functional connectivity; functional localization; language network; lateralization; multiple demand network.
Conflict of interest statement
Competing Interest Statement: Dr. Kiran is a scientific advisor for Constant Therapy Health, but there is no overlap between this role and the submitted investigation. The other authors report no conflicts.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
