This is a preprint.
Cellular and molecular mechanisms that shape the development and evolution of tail vertebral proportion in mice and jerboas
- PMID: 39484405
- PMCID: PMC11527341
- DOI: 10.1101/2024.10.25.620311
Cellular and molecular mechanisms that shape the development and evolution of tail vertebral proportion in mice and jerboas
Update in
-
Cellular and genetic mechanisms that shape the development and evolution of tail vertebral proportion in mice and jerboas.Nat Commun. 2025 Oct 10;16(1):9014. doi: 10.1038/s41467-025-63606-9. Nat Commun. 2025. PMID: 41073372 Free PMC article.
Abstract
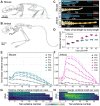
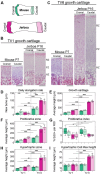
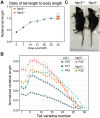
Despite the functional importance of the vertebral skeleton, little is known about how individual vertebrae elongate or achieve disproportionate lengths as in the giraffe neck. Rodent tails are an abundantly diverse and more tractable system to understand mechanisms of vertebral growth and proportion. In many rodents, disproportionately long mid-tail vertebrae form a 'crescendo-decrescendo' of lengths in the tail series. In bipedal jerboas, these vertebrae grow exceptionally long such that the adult tail is 1.5x the length of a mouse tail, relative to body length, with four fewer vertebrae. How do vertebrae with the same regional identity elongate differently from their neighbors to establish and diversify adult proportion? Here, we find that vertebral lengths are largely determined by differences in growth cartilage height and the number of cells progressing through endochondral ossification. Hypertrophic chondrocyte size, a major contributor to differential elongation in mammal limb bones, differs only in the longest jerboa mid-tail vertebrae where they are exceptionally large. To uncover candidate molecular mechanisms of disproportionate vertebral growth, we performed intersectional RNA-Seq of mouse and jerboa tail vertebrae with similar and disproportionate elongation rates. Many regulators of posterior axial identity and endochondral elongation are disproportionately differentially expressed in jerboa vertebrae. Among these, the inhibitory natriuretic peptide receptor C (NPR3) appears in multiple studies of rodent and human skeletal proportion suggesting it refines local growth rates broadly in the skeleton and broadly in mammals. Consistent with this hypothesis, NPR3 loss of function mice have abnormal tail and limb proportions. Therefore, in addition to genetic components of the complex process of vertebral evolution, these studies reveal fundamental mechanisms of skeletal growth and proportion.
Figures

References
-
- Cooper K.L., and Tabin C.J. (2009). Developmental patterning of the limb skeleton. In The Skeletal System (Cold Spring Harbor Press; ), p. 39.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
