This is a preprint.
Epigenetic control of Topoisomerase 1 activity presents a cancer vulnerability
- PMID: 39484415
- PMCID: PMC11526978
- DOI: 10.1101/2024.10.22.619113
Epigenetic control of Topoisomerase 1 activity presents a cancer vulnerability
Update in
-
Epigenetic control of topoisomerase 1 activity presents a cancer vulnerability.Nat Commun. 2025 Aug 12;16(1):7458. doi: 10.1038/s41467-025-62598-w. Nat Commun. 2025. PMID: 40796804 Free PMC article.
Abstract
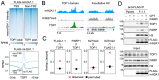
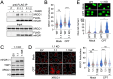
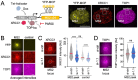
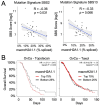
DNA transactions introduce torsional constraints that pose an inherent risk to genome integrity. While topoisomerase 1 (TOP1) activity is essential for removing DNA supercoiling, aberrant stabilization of TOP1:DNA cleavage complexes (TOP1ccs) can result in cytotoxic DNA lesions. What protects genomic hot spots of topological stress from aberrant TOP1 activity remains unknown. Here, we identify chromatin context as an essential means to coordinate TOP1cc resolution. Through its ability to bind poly(ADP-ribose) (PAR), a protein modification required for TOP1cc repair, the histone variant macroH2A1.1 establishes a TOP1-permissive chromatin environment, while the alternatively spliced macroH2A1.2 isoform is unable to bind PAR or protect from TOP1ccs. By visualizing transcription-induced topological stress in single cells, we find that macroH2A1.1 facilitates PAR-dependent recruitment of the TOP1cc repair effector XRCC1 to protect from ssDNA damage. Impaired macroH2A1.1 splicing, a frequent cancer feature, was predictive of increased sensitivity to TOP1 poisons in a pharmaco-genomic screen in breast cancer cells, and macroH2A1.1 inactivation mirrored this effect. Consistent with this, low macroH2A1.1 expression correlated with improved survival in cancer patients treated with TOP1 inhibitors. We propose that macroH2A1 alternative splicing serves as an epigenetic modulator of TOP1-associated genome maintenance and a potential cancer vulnerability.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
