This is a preprint.
ACSS2 regulates ferroptosis in an E2F1-dependent manner in breast cancer brain metastatic cells
- PMID: 39484430
- PMCID: PMC11526985
- DOI: 10.1101/2024.10.18.619082
ACSS2 regulates ferroptosis in an E2F1-dependent manner in breast cancer brain metastatic cells
Abstract
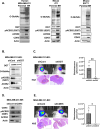
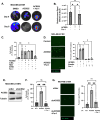
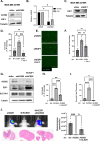
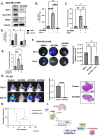
Brain metastasis diagnosis in breast cancer patients is considered an end-stage event. The median survival after diagnosis is measured in months, thus there is an urgent need to develop novel treatment strategies. Breast cancers that metastasize to the brain must adapt to the unique brain environment and are highly dependent on acetate metabolism for growth and survival. However, the signaling pathways that regulate survival in breast cancer brain metastatic (BCBM) tumors are not known. Primary brain tumor cells can convert acetate to acetyl-CoA via phosphorylation of acetyl-CoA synthetase 2 (ACSS2) by the cyclin-dependent kinase-5 (CDK5) regulated by the nutrient sensor O-GlcNAc transferase (OGT). Here, we show that breast cancer cells selected to metastasize to the brain contain increased levels of O-GlcNAc, OGT and ACSS2-Ser267 phosphorylation compared to parental breast cancer cells. Moreover, OGT and CDK5 are required for breast cancer cell growth in the brain parenchyma in vivo. Importantly, ACSS2 and ACSS2-S267D phospho-mimetic mutant are critical for in vivo breast cancer growth in the brain but not in the mammary fat pad. Mechanistically, we show that ACSS2 regulates BCBM cell survival by suppressing ferroptosis via regulation of E2F1-mediated expression of anti-ferroptotic proteins SLC7A11 and GPX4. Lastly, we show treatment with a novel brain-permeable small molecule ACSS2 inhibitor induced ferroptosis and reduced BCBM growth ex vivo and in vivo. These results suggest a crucial role for ACSS2 in protecting from ferroptosis in breast cancer brain metastatic cells and suggests that breast cancer brain metastatic cells may be susceptible to ferroptotic inducers.
Keywords: ACSS2; CDK5; E2F1; GPX4; O-GlcNAc; OGT; SLC7A11; acetate; acetyl-CoA; brain metastasis; breast cancer; cancer; ferroptosis; hexosamine; lipids; metabolism.
Conflict of interest statement
Competing Interests. A.D., L.C. and M.J.R. are inventors on patents involving ACSS2 inhibitor AD-5584.
Figures


References
-
- Sokoloff L., Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J Cereb Blood Flow Metab, 1981. 1(1): p. 7–36. - PubMed
-
- Edmond J., et al. , Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res, 1987. 18(4): p. 551–61. - PubMed
-
- Dienel G.A., Brain Glucose Metabolism: Integration of Energetics with Function. Physiol Rev, 2019. 99(1): p. 949–1045. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
