This is a preprint.
Myeloid-derived β-hexosaminidase is essential for neuronal health and lysosome function: implications for Sandhoff disease
- PMID: 39484433
- PMCID: PMC11526954
- DOI: 10.1101/2024.10.21.619538
Myeloid-derived β-hexosaminidase is essential for neuronal health and lysosome function: implications for Sandhoff disease
Abstract
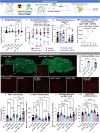
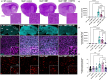
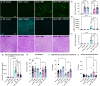
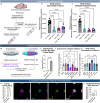
Lysosomal storage disorders (LSDs) are a large disease class involving lysosomal dysfunction, often resulting in neurodegeneration. Sandhoff disease (SD) is an LSD caused by a deficiency in the β subunit of the β-hexosaminidase enzyme (Hexb). Although Hexb expression in the brain is specific to microglia, SD primarily affects neurons. To understand how a microglial gene is involved in maintaining neuronal homeostasis, we demonstrated that β-hexosaminidase is secreted by microglia and integrated into the neuronal lysosomal compartment. To assess therapeutic relevance, we treated SD mice with bone marrow transplant and colony stimulating factor 1 receptor inhibition, which broadly replaced Hexb -/- microglia with Hexb-sufficient cells. This intervention reversed apoptotic gene signatures, improved behavior, restored enzymatic activity and Hexb expression, ameliorated substrate accumulation, and normalized neuronal lysosomal phenotypes. These results underscore the critical role of myeloid-derived β-hexosaminidase in neuronal lysosomal function and establish microglial replacement as a potential LSD therapy.
Conflict of interest statement
DECLARATION OF INTERESTS Kim N. Green is on the scientific advisory board of Ashvattha Therapeutics, Inc. All other authors declare no conflict of interest.
Figures



References
-
- Platt F. M., d’Azzo A., Davidson B. L., Neufeld E. F. & Tifft C. J. Lysosomal storage diseases. Nat. Rev. Dis. Primer 4, 1–25 (2018). - PubMed
-
- Fuller M., Meikle P. J. & Hopwood J. J. Epidemiology of lysosomal storage diseases: an overview. in Fabry Disease: Perspectives from 5 Years of FOS (eds. Mehta A., Beck M. & Sunder-Plassmann G.) (Oxford PharmaGenesis, Oxford, 2006). - PubMed
-
- Huang J. Q. et al. Apoptotic cell death in mouse models of GM2 gangliosidosis and observations on human Tay-Sachs and Sandhoff diseases. Hum. Mol. Genet. 6, 1879–1885 (1997). - PubMed
-
- Conzelmann E. & Sandhoff K. Purification and characterization of an activator protein for the degradation of glycolipids GM2 and GA2 by hexosaminidase A. Hoppe. Seylers Z. Physiol. Chem. 360, 1837–1849 (1979). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
