This is a preprint.
Convergent olfactory circuits for courtship in Drosophila revealed by ds-Tango
- PMID: 39484479
- PMCID: PMC11527207
- DOI: 10.1101/2024.10.23.619891
Convergent olfactory circuits for courtship in Drosophila revealed by ds-Tango
Abstract
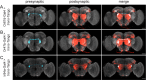
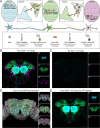
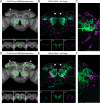
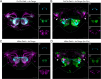
Animals exhibit sex-specific behaviors that are governed by sexually dimorphic circuits. One such behavior in male Drosophila melanogaster, courtship, is regulated by various sensory modalities, including olfaction. Here, we reveal how sexually dimorphic olfactory pathways in male flies converge at the third-order, onto lateral horn output neurons, to regulate courtship. To achieve this, we developed ds-Tango, a modified version of the monosynaptic tracing and manipulation tool trans-Tango. In ds-Tango, two distinct configurations of trans-Tango are positioned in series, thus providing selective genetic access not only to the monosynaptic partners of starter neurons but also to their disynaptic connections. Using ds-Tango, we identified a node of convergence for three sexually dimorphic olfactory pathways. Silencing this node results in deficits in sex recognition of potential partners. Our results identify lateral horn output neurons required for proper courtship behavior in male flies and establish ds-Tango as a tool for disynaptic circuit tracing.
Keywords: Drosophila; courtship; disynaptic tracing; ds-Tango; lateral horn; neural circuit.
Figures

References
-
- Miles M.C., and Fuxjager M.J. (2018). Animal choreography of song and dance: a case study in the Montezuma oropendola,. Anim Behav 140, 99–107. 10.1016/j.anbehav.2018.04.006. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
