This is a preprint.
Arc mediates intercellular tau transmission via extracellular vesicles
- PMID: 39484489
- PMCID: PMC11526995
- DOI: 10.1101/2024.10.22.619703
Arc mediates intercellular tau transmission via extracellular vesicles
Abstract
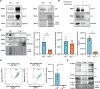
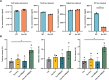
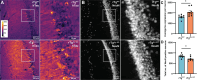
Intracellular neurofibrillary tangles that consist of misfolded tau protein1 cause neurodegeneration in Alzheimer's disease (AD) and frontotemporal dementia (FTD). Tau pathology spreads cell-to-cell2 but the exact mechanisms of tau release and intercellular transmission remain poorly defined. Tau is released from neurons as free protein or in extracellular vesicles (EVs)3-5 but the role of these different release mechanisms in intercellular tau transmission is unclear. Here, we show that the neuronal gene Arc is critical for packaging tau into EVs. Brain EVs purified from human tau (hTau) transgenic rTg4510 mice (rTgWT) contain high levels of hTau that are capable of seeding tau pathology. In contrast, EVs purified from rTgWT crossed with Arc knock-out mice (rTgArc KO) have significantly less hTau and cannot seed tau aggregation. Arc facilitates the release of hTau in EVs produced via the I-BAR protein IRSp53, but not free tau. Arc protein directly binds hTau to form a fuzzy complex that we identified in both mouse and human brain tissue. We find that pathological intracellular hTau accumulates in neurons in rTgArc KO mice, which correlates with accelerated neuron loss in the hippocampus. Finally, we find that intercellular tau transmission is significantly abrogated in Arc KO mice. We conclude that Arc-dependent release of tau in EVs plays a significant role in intracellular tau elimination and intercellular tau transmission.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
