This is a preprint.
Oncogenic Alterations, Race, and Survival in US Veterans with Metastatic Prostate Cancer Undergoing Somatic Tumor Next Generation Sequencing
- PMID: 39484518
- PMCID: PMC11527339
- DOI: 10.1101/2024.10.24.620071
Oncogenic Alterations, Race, and Survival in US Veterans with Metastatic Prostate Cancer Undergoing Somatic Tumor Next Generation Sequencing
Update in
-
Somatic Tumor Next-Generation Sequencing in US Veterans With Metastatic Prostate Cancer.JAMA Netw Open. 2025 May 1;8(5):e259119. doi: 10.1001/jamanetworkopen.2025.9119. JAMA Netw Open. 2025. PMID: 40354055 Free PMC article.
Abstract
Purpose: National guidelines recommend next generation sequencing (NGS) of tumors in patients diagnosed with metastatic prostate cancer (mPCa) to identify potential actionable alterations. We sought to describe the spectrum and frequency of alterations in PCa-related genes and pathways, as well as associations with self-identified race/ethnicity, and overall survival in US Veterans.
Patients and methods: This retrospective cohort study included Non-Hispanic Black (NHB) and Non-Hispanic white (NHW) Veterans with mPCa who obtained NGS through the Veterans Affairs National Precision Oncology Program. 45 genes in seven canonical or targetable mPCa pathways were evaluated in addition to TMB and MSI status. Multivariable logistic regression evaluated associations between race/ethnicity and genomic alteration frequencies. Cox proportional hazards models were used to determine associations between race/ethnicity, specific gene/pathway alteration, and overall survival.
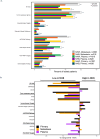
Results: 5,015 Veterans with mPCa who had NGS conducted were included (1,784 NHB, 3,231 NHW). NHB Veterans were younger, had higher PSA at diagnosis, were less likely to report Agent Orange exposure, and resided in more deprived neighborhoods compared to NHW Veterans. Nine of the top ten most commonly altered genes were the same in NHB v NHW Veterans; however, the frequencies of alterations varied by race/ethnicity. NHB race/ethnicity was associated with higher odds of genomic alterations in SPOP (OR 1.7 [1.2-2.6]) as well as immunotherapy targets (OR 1.7 [1.1-2.7]) including MSI high status (OR 3.1 [1.1-9.4]). Furthermore, NHB race/ethnicity was significantly associated with lower odds of genomic alterations in the AKT/PI3K pathway (OR 0.6 [0.4-0.7]), AR axis (OR 0.7 [0.5-0.9]), and tumor suppressor genes (OR 0.7 [0.5-0.8]). Cox proportional hazards modelling stratified by race/ethnicity demonstrated alterations in tumor suppressor genes including TP53 were associated with shorter OS in both NHB (HR 1.54 [1.13-2.11] and NHW individuals (HR 1.52 [1.25-1.85]).
Conclusion: In the equal access VA healthcare setting, Veterans undergoing NGS for mPCa exhibited differences in alteration frequencies in both actionable and non-actionable pathways that may be associated with survival. This analysis affirms the utility of genomic testing for identifying candidates irrespective of race/ethnicity for precision oncology treatments, which could contribute to equitable outcomes in patients with mPCa.
Conflict of interest statement
Conflict of Interest Disclosures: Dr. Valle reported receiving funding from The Bristol Myers Squibb Foundation, Mike Slive Foundation for Prostate Cancer Research, the Parker Institute for Cancer Immunotherapy, and the Jonsson Comprehensive Cancer Center. Dr Rettig reported receiving grants from the Prostate Cancer Foundation during the conduct of the study; receiving personal fees from Bayer, Janssen, Inmune Bio, and Myovant; grants from Merck, Progenics, Clovis, ORIC, Pfizer, Novartis, and Amgen; and nonfinancial support from Merck, Lantheus, Clovis, ORIC, Novartis, and Amgen outside the submitted work; and holding a patent, issued, for Inhibitors of the N-terminal Domain of the Androgen Receptor. Dr. Chatwal reported being on the Merck advisory board. Dr. Kelley reported receiving research funding from Regeneron, and Bristol Myers Squibb. Dr Nickols reported receiving grants from the Prostate Cancer Foundation during the conduct of the study; grants from Janssen, Bayer, and Lantheus outside the submitted work; and consulting fee from PrimeFour outside the submitted work. Dr. Garraway reported receiving research funding from the Prostate Cancer Foundation, Jean Perkins Foundation, and STOP Cancer Foundation. Dr. Yamoah reported receiving research funding from the Prostate Cancer Foundation. Dr. Maxwell reported receiving research funding from Prostate Cancer Foundation, Burroughs Welcome Foundation and the Basser Center for BRCA at the University of Pennsylvania. No other disclosures were reported.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
