This is a preprint.
Phenotypic landscape of a fungal meningitis pathogen reveals its unique biology
- PMID: 39484549
- PMCID: PMC11526942
- DOI: 10.1101/2024.10.22.619677
Phenotypic landscape of a fungal meningitis pathogen reveals its unique biology
Update in
-
Phenotypic landscape of an invasive fungal pathogen reveals its unique biology.Cell. 2025 Jul 24;188(15):4003-4024.e24. doi: 10.1016/j.cell.2025.05.017. Epub 2025 Jun 11. Cell. 2025. PMID: 40505656
Abstract
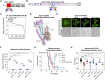
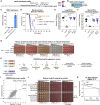
Cryptococcus neoformans is the most common cause of fungal meningitis and the top-ranked W.H.O. priority fungal pathogen. Only distantly related to model fungi, C. neoformans is also a powerful experimental system for exploring conserved eukaryotic mechanisms lost from specialist model yeast lineages. To decipher its biology globally, we constructed 4328 gene deletions and measured-with exceptional precision--the fitness of each mutant under 141 diverse growth-limiting in vitro conditions and during murine infection. We defined functional modules by clustering genes based on their phenotypic signatures. In-depth studies leveraged these data in two ways. First, we defined and investigated new components of key signaling pathways, which revealed animal-like pathways/components not predicted from studies of model yeasts. Second, we identified environmental adaptation mechanisms repurposed to promote mammalian virulence by C. neoformans, which lacks a known animal reservoir. Our work provides an unprecedented resource for deciphering a deadly human pathogen.
Figures





References
-
- Rajasingham R., Govender N.P., Jordan A., Loyse A., Shroufi A., Denning D.W., Meya D.B., Chiller T.M., and Boulware D.R. (2022). The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect. Dis. 22, 1748–1755. 10.1016/s1473-3099(22)00499-6. - DOI - PMC - PubMed
-
- WHO/UCN/HHS/SIA/2023.05 (2024). South Africa HIV Country Profile 2023.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
