This is a preprint.
Bioorthogonal cyclopropenones for investigating RNA structure
- PMID: 39484557
- PMCID: PMC11527001
- DOI: 10.1101/2024.10.22.619649
Bioorthogonal cyclopropenones for investigating RNA structure
Update in
-
Bioorthogonal Cyclopropenones for Investigating RNA Structure.ACS Chem Biol. 2024 Dec 20;19(12):2406-2411. doi: 10.1021/acschembio.4c00633. Epub 2024 Dec 6. ACS Chem Biol. 2024. PMID: 39641920 Free PMC article.
Abstract
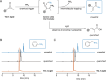
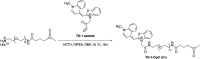
RNA sequences encode secondary and tertiary structures that impact protein production and other cellular processes. Misfolded RNAs can also potentiate disease, but the complete picture is lacking. To establish more comprehensive and accurate RNA structure-function relationships, new methods are needed to interrogate RNA and trap native conformations in cellular environments. Existing tools primarily rely on electrophiles that are constitutively "on" or triggered by UV light, often resulting in high background reactivity. We developed an alternative, chemically triggered approach to crosslink RNAs using bioorthogonal cyclopropenones (CpOs). These reagents selectively react with phosphines to provide ketenes-electrophiles that can trap neighboring nucleophiles to forge covalent crosslinks. As proof-of-concept, we synthesized a panel of CpOs and appended them to thiazole orange (TO-1). The TO-1 conjugates bound selectively to a model RNA aptamer (Mango) with nanomolar affinity, confirmed by fluorescence turn-on. After phosphine administration, covalent crosslinks were formed between the CpO probes and RNA. The degree of crosslinking was both time and dose-dependent. We further applied the chemically triggered tools to model RNAs in biologically relevant conditions. Collectively, this work expands the toolkit of probes for studying RNA and its native conformations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Alberts B.; Johnson A.; Lewis J.; Raff M.; Roberts K.; Walter P. From RNA to Protein. In Molecular Biology of the Cell. 4th edition; Garland Science, 2002.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
