This is a preprint.
Topography of putative bidirectional interaction between hippocampal sharp wave ripples and neocortical slow oscillations
- PMID: 39484611
- PMCID: PMC11526890
- DOI: 10.1101/2024.10.23.619879
Topography of putative bidirectional interaction between hippocampal sharp wave ripples and neocortical slow oscillations
Update in
-
Topography of putative bi-directional interaction between hippocampal sharp-wave ripples and neocortical slow oscillations.Neuron. 2025 Mar 5;113(5):754-768.e9. doi: 10.1016/j.neuron.2024.12.019. Epub 2025 Jan 27. Neuron. 2025. PMID: 39874961
Abstract
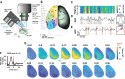
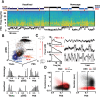
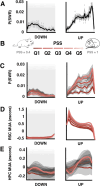
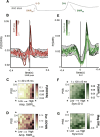
Systems consolidation relies on coordination between hippocampal sharp-wave ripples (SWRs) and neocortical UP/DOWN states during sleep. However, whether this coupling exists across neocortex and the mechanisms enabling it remain unknown. By combining electrophysiology in mouse hippocampus (HPC) and retrosplenial cortex (RSC) with widefield imaging of dorsal neocortex, we found spatially and temporally precise bidirectional hippocampo-neocortical interaction. HPC multi-unit activity and SWR probability was correlated with UP/DOWN states in mouse default mode network, with highest modulation by RSC in deep sleep. Further, some SWRs were preceded by the high rebound excitation accompanying DMN DOWN→UP transitions, while large-amplitude SWRs were often followed by DOWN states originating in RSC. We explain these electrophysiological results with a model in which HPC and RSC are weakly coupled excitable systems capable of bi-directional perturbation and suggest RSC may act as a gateway through which SWRs can perturb downstream cortical regions via cortico-cortical propagation of DOWN states.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures



References
-
- McClelland J. L., McNaughton B. L., and O’Reilly R. C., “Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory.,” Psychol. Rev., vol. 102, no. 3, pp. 419–457, 1995, doi: 10.1037/0033-295X.102.3.419. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
