This is a preprint.
Optical characterization of molecular interaction strength in protein condensates
- PMID: 39484615
- PMCID: PMC11526858
- DOI: 10.1101/2024.03.19.585750
Optical characterization of molecular interaction strength in protein condensates
Update in
-
Optical characterization of molecular interaction strength in protein condensates.Mol Biol Cell. 2024 Dec 1;35(12):ar154. doi: 10.1091/mbc.E24-03-0128. Epub 2024 Nov 13. Mol Biol Cell. 2024. PMID: 39535884 Free PMC article.
Abstract
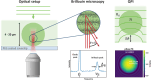
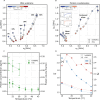
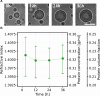
Biomolecular condensates have been identified as a ubiquitous means of intracellular organization, exhibiting very diverse material properties. However, techniques to characterize these material properties and their underlying molecular interactions are scarce. Here, we introduce two optical techniques - Brillouin microscopy and quantitative phase imaging (QPI) - to address this scarcity. We establish Brillouin shift and linewidth as measures for average molecular interaction and dissipation strength, respectively, and we used QPI to obtain the protein concentration within the condensates. We monitored the response of condensates formed by FUS and by the low-complexity domain of hnRNPA1 (A1-LCD) to altering temperature and ion concentration. Conditions favoring phase separation increased Brillouin shift, linewidth, and protein concentration. In comparison to solidification by chemical crosslinking, the ion-dependent aging of FUS condensates had a small effect on the molecular interaction strength inside. Finally, we investigated how sequence variations of A1-LCD, that change the driving force for phase separation, alter the physical properties of the respective condensates. Our results provide a new experimental perspective on the material properties of protein condensates. Robust and quantitative experimental approaches such as the presented ones will be crucial for understanding how the physical properties of biological condensates determine their function and dysfunction.
Conflict of interest statement
Conflicts of interest RS is an employee of the company CellSense GmbH, which develops commercial Brillouin microscopes. The other authors declare no competing interests.
Figures


References
-
- Brangwynne Clifford P., Tompa Peter, and Pappu Rohit V. “Polymer physics of intracellular phase transitions”. In: Nature Physics 11.11 (2015), pp. 899–904. doi: 10.1038/nphys3532. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
