Activation of STAT3-mediated ciliated cell survival protects against severe infection by respiratory syncytial virus
- PMID: 39484716
- PMCID: PMC11527452
- DOI: 10.1172/JCI183978
Activation of STAT3-mediated ciliated cell survival protects against severe infection by respiratory syncytial virus
Abstract
Respiratory syncytial virus (RSV) selectively targets ciliated cells in human bronchial epithelium and can cause bronchiolitis and pneumonia, mostly in infants. To identify molecular targets of intervention during RSV infection in infants, we investigated how age regulates RSV interaction with the bronchial epithelium barrier. Employing precision-cut lung slices and air-liquid interface cultures generated from infant and adult human donors, we found robust RSV virus spread and extensive apoptotic cell death only in infant bronchial epithelium. In contrast, adult bronchial epithelium showed no barrier damage and limited RSV infection. Single nuclear RNA-Seq revealed age-related insufficiency of an antiapoptotic STAT3 activation response to RSV infection in infant ciliated cells, which was exploited to facilitate virus spread via the extruded apoptotic ciliated cells carrying RSV. Activation of STAT3 and blockade of apoptosis rendered protection against severe RSV infection in infant bronchial epithelium. Lastly, apoptotic inhibitor treatment of a neonatal mouse model of RSV infection mitigated infection and inflammation in the lung. Taken together, our findings identify a STAT3-mediated antiapoptosis pathway as a target to battle severe RSV disease in infants.
Keywords: Apoptosis; Infectious disease.
Conflict of interest statement
Figures
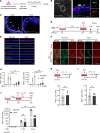
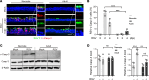
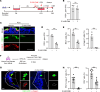
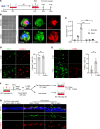
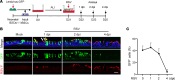


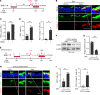
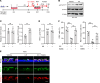
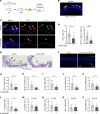
Comment in
- Enhanced susceptibility of pediatric airway epithelium to respiratory syncytial virus infection doi: 10.1172/JCI185689
References
-
- Wang X, et al. Global disease burden of and risk factors for acute lower respiratory infections caused by respiratory syncytial virus in preterm infants and young children in 2019: a systematic review and meta-analysis of aggregated and individual participant data. Lancet. 2024;403(10433):1241–1253. doi: 10.1016/S0140-6736(24)00138-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

