A scenario for an evolutionary selection of ageing
- PMID: 39485277
- PMCID: PMC11530237
- DOI: 10.7554/eLife.92914
A scenario for an evolutionary selection of ageing
Abstract
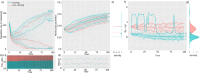
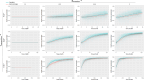
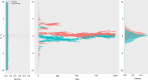
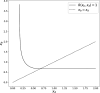
Signs of ageing become apparent only late in life, after organismal development is finalized. Ageing, most notably, decreases an individual's fitness. As such, it is most commonly perceived as a non-adaptive force of evolution and considered a by-product of natural selection. Building upon the evolutionarily conserved age-related Smurf phenotype, we propose a simple mathematical life-history trait model in which an organism is characterized by two core abilities: reproduction and homeostasis. Through the simulation of this model, we observe (1) the convergence of fertility's end with the onset of senescence, (2) the relative success of ageing populations, as compared to non-ageing populations, and (3) the enhanced evolvability (i.e. the generation of genetic variability) of ageing populations. In addition, we formally demonstrate the mathematical convergence observed in (1). We thus theorize that mechanisms that link the timing of fertility and ageing have been selected and fixed over evolutionary history, which, in turn, explains why ageing populations are more evolvable and therefore more successful. Broadly speaking, our work suggests that ageing is an adaptive force of evolution.
Keywords: adaptability; ageing evolution; differential inclusion; evolutionary biology; none; two phases model.
Plain language summary
It is a question as old as Darwin’s theory of evolution itself: how is ageing affected by natural selection? The prevailing view is that the process of biological ageing is not adaptive and therefore not directly subject to selection pressures. Take for example a gene causing a fatal disease late after an average individual had reproduced, thus being passed on to the next generation despite its detriment to the individual. This suggests that natural selection acts less strongly on such genes, which can therefore accumulate and cause aging if they do not impact an organism’s reproductive fitness earlier in life. However, many studies have shown that specific genes control an animal’s lifespan and the onset of ageing through evolutionarily conserved mechanisms. For example, in fruit flies, aging can be categorised into two distinct phases determined by the manifestation of the so-called Smurf phenotype associated with accelerated signs of ageing and an increased risk of death. A pattern where the offspring of older parents live less long than those of younger parents has also been observed across species, also known as the Lansing effect. In this case, ageing can affect the reproductive success of future generations and can therefore be subject to selection pressures. Roget et al. looked at the trade-offs between an individual’s reproduction and homeostasis using a mathematical model to address whether the distinct phases of aging – as seen in the Smurf phenotype – can appear and be maintained throughout evolution. Using a mathematical model, Roget et al. simulated individuals possessing only one copy of two genes. One controls the duration of reproductive ability, and the other defines the age at which the risk of death becomes non-zero. This revealed that a simple hypothetical haploid and asexually reproducing system can evolve a life history separated into two phases in the computer simulations. Interestingly, the modelled organisms evolved in a way that the duration of reproduction exceeded the homeostatic maintenance duration. This generated a phase where individuals are capable of reproduction with a high risk of death, similar to the previously described Smurf phase. Roget et al. observed that aging populations showed a lower risk of extinction than non-aging ones, as well as an increased genetic variability of the offspring. The apparent benefits of ageing in this model imply that ageing can be an adaptive force of evolution and subject to positive selection or, at least less negative selection than expected. This minimal model helps explain trade-offs between reproduction and homeostatic maintenance during evolution. Further work may include parameters such as sexual reproduction and multiple gene copies.
© 2024, Roget et al.
Conflict of interest statement
TR, CM, PJ, SM, MR No competing interests declared
Figures





Update of
- doi: 10.1101/2022.03.11.483978
- doi: 10.7554/eLife.92914.1
- doi: 10.7554/eLife.92914.2
References
-
- Arslan RC, Willführ KP, Frans EM, Verweij KJH, Bürkner PC, Myrskylä M, Voland E, Almqvist C, Zietsch BP, Penke L. Older fathers’ children have lower evolutionary fitness across four centuries and in four populations. Proceedings. Biological Sciences. 2017;284:20171562. doi: 10.1098/rspb.2017.1562. - DOI - PMC - PubMed
-
- Burke MK, Barter TT, Cabral LG, Kezos JN, Phillips MA, Rutledge GA, Phung KH, Chen RH, Nguyen HD, Mueller LD, Rose MR. Rapid divergence and convergence of life-history in experimentally evolved Drosophila melanogaster. Evolution; International Journal of Organic Evolution. 2016;70:2085–2098. doi: 10.1111/evo.13006. - DOI - PubMed
-
- Cansell C, Goepp V, Bain F, Todd N, Douard V, Monnoye M, Sanchez C, Pietrancosta N, Rovere C, Denis RG, Luquet S, Rera M. 2023. Two phases model of ageing in mice: towards a better identification of age-related and late-life metabolic decline [Registered Report Stage 1 Protocol] figshare. - DOI
-
- Charlesworth B. Evolution in Age-Structured Populations. Cambridge University Press; 1994. - DOI
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

