Calcineurin inhibition enhances Caenorhabditis elegans lifespan by defecation defects-mediated calorie restriction and nuclear hormone signaling
- PMID: 39485281
- PMCID: PMC11530235
- DOI: 10.7554/eLife.89572
Calcineurin inhibition enhances Caenorhabditis elegans lifespan by defecation defects-mediated calorie restriction and nuclear hormone signaling
Abstract
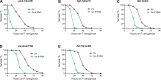
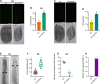
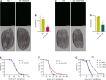
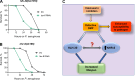
Calcineurin is a highly conserved calcium/calmodulin-dependent serine/threonine protein phosphatase with diverse functions. Inhibition of calcineurin is known to enhance the lifespan of Caenorhabditis elegans through multiple signaling pathways. Aiming to study the role of calcineurin in regulating innate immunity, we discover that calcineurin is required for the rhythmic defecation motor program (DMP) in C. elegans. Calcineurin inhibition leads to defects in the DMP, resulting in intestinal bloating, rapid colonization of the gut by bacteria, and increased susceptibility to bacterial infection. We demonstrate that intestinal bloating caused by calcineurin inhibition mimics the effects of calorie restriction, resulting in enhanced lifespan. The TFEB ortholog, HLH-30, is required for lifespan extension mediated by calcineurin inhibition. Finally, we show that the nuclear hormone receptor, NHR-8, is upregulated by calcineurin inhibition and is necessary for the increased lifespan. Our studies uncover a role for calcineurin in the C. elegans DMP and provide a new mechanism for calcineurin inhibition-mediated longevity extension.
Keywords: C. elegans; Intestinal bloating; calorie restriction; cell biology; genetics; genomics; hlh-30; longevity; nhr-8; tax-6.
Plain language summary
Many research efforts currently focus on identifying the dietary, pharmacological or genetic interventions that could help to prolong life. In the process, these investigations often uncover complex or even unexpected relationships between a range of physiological processes. The link between longevity and the immune system, for example, is yet to be fully understood. To explore these dynamics, Das et al. focused on calcineurin, an enzyme present in organisms across the tree of life. In humans, calcineurin is known to regulate a set of proteins essential for the immune response; these proteins are absent in the microscopic worm Caenorhabditis elegans, in which inhibiting calcineurin extends lifespan. Investigating how calcineurin inhibition impacts the immune system of C. elegans therefore presents a unique opportunity to better understand the complex links between immunity and longevity. Experiments conducted on worms genetically modified to lack calcineurin showed that these animals lived longer than their ‘normal’ counterparts, but that they were also more susceptible to infection when exposed to a harmful species of bacteria. Further experiments showed that the enzyme was crucial for regulating defecation in C. elegans. Without calcineurin, the worms became bloated and constipated; they could not properly eliminate bacteria, which could then proliferate in the digestive system and cause issues. However, intestinal bloating also activated signalling pathways normally triggered by calorie restriction – an intervention well-known for extending the lifespan of various species. Taken together, the findings by Das et al. help explain why calcineurin inhibition in C. elegans leads to opposite effects on longevity and resistance to infection. They also align with a recent body of work showing the profound effect of gut bloating on food-seeking behaviors, immunity and lifespan. Further investigations into these mechanisms may one day uncover new ways to improve human health.
© 2023, Das et al.
Conflict of interest statement
PD, AA, JS No competing interests declared
Figures












Update of
- doi: 10.1101/2023.06.21.545873
- doi: 10.7554/eLife.89572.1
- doi: 10.7554/eLife.89572.2
References
-
- Amrit FRG, Naim N, Ratnappan R, Loose J, Mason C, Steenberge L, McClendon BT, Wang G, Driscoll M, Yanowitz JL, Ghazi A. The longevity-promoting factor, TCER-1, widely represses stress resistance and innate immunity. Nature Communications. 2019;10:3042. doi: 10.1038/s41467-019-10759-z. - DOI - PMC - PubMed
-
- Aveleira CA, Botelho M, Carmo-Silva S, Pascoal JF, Ferreira-Marques M, Nóbrega C, Cortes L, Valero J, Sousa-Ferreira L, Álvaro AR, Santana M, Kügler S, Pereira de Almeida L, Cavadas C. Neuropeptide Y stimulates autophagy in hypothalamic neurons. PNAS. 2015;112:E1642–E1651. doi: 10.1073/pnas.1416609112. - DOI - PMC - PubMed
-
- Bandyopadhyay J, Lee J, Lee J, Lee JI, Yu JR, Jee C, Cho JH, Jung S, Lee MH, Zannoni S, Singson A, Kim DH, Koo HS, Ahnn J. Calcineurin, a calcium/calmodulin-dependent protein phosphatase, is involved in movement, fertility, egg laying, and growth in Caenorhabditis elegans. Molecular Biology of the Cell. 2002;13:3281–3293. doi: 10.1091/mbc.e02-01-0005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- P40 OD010440/OD/NIH HHS/United States
- 37/1741/23/EMR-II/Council of Scientific and Industrial Research, India
- SRG/2020/000022/Science and Engineering Research Board
- CRG/2023/001136/Science and Engineering Research Board
- HRD-17011/2/2023-HRD-DBT/Department of Biotechnology, Ministry of Science and Technology, India
LinkOut - more resources
Full Text Sources
Medical
Research Materials

