Auto-Abs neutralizing type I IFNs in patients with severe Powassan, Usutu, or Ross River virus disease
- PMID: 39485284
- PMCID: PMC11533500
- DOI: 10.1084/jem.20240942
Auto-Abs neutralizing type I IFNs in patients with severe Powassan, Usutu, or Ross River virus disease
Abstract
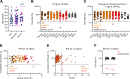
Arboviral diseases are a growing global health concern. Pre-existing autoantibodies (auto-Abs) neutralizing type I interferons (IFNs) can underlie encephalitis due to West Nile virus (WNV) (∼40% of patients) and tick-borne encephalitis (TBE, due to TBE virus [TBEV]) (∼10%). We report here that these auto-Abs can also underlie severe forms of rarer arboviral infections. Auto-Abs neutralizing high concentrations of IFN-α2, IFN-β, and/or IFN-ω are present in the single case of severe Powassan virus (POWV) encephalitis studied, two of three cases of severe Usutu virus (USUV) infection studied, and the most severe of 24 cases of Ross River virus (RRV) disease studied. These auto-Abs are not found in any of the 137 individuals with silent or mild infections with these three viruses. Thus, auto-Abs neutralizing type I IFNs underlie an increasing list of severe arboviral diseases due to Flaviviridae (WNV, TBEV, POWV, USUV) or Togaviridae (RRV) viruses transmitted to humans by mosquitos (WNV, USUV, RRV) or ticks (TBEV, POWV).
© 2024 Gervais et al.
Conflict of interest statement
Disclosures: M. Agudelo reported personal fees from Boehringer Ingelheim outside the submitted work. No other disclosures were reported.
Figures


References
-
- Abers, M.S., Rosen L.B., Delmonte O.M., Shaw E., Bastard P., Imberti L., Quaresima V., Biondi A., Bonfanti P., Castagnoli R., et al. . 2021. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol. Cell Biol. 99:917–921. 10.1111/imcb.12495 - DOI - PMC - PubMed
-
- Abolhassani, H., Landegren N., Bastard P., Materna M., Modaresi M., Du L., Aranda-Guillén M., Sardh F., Zuo F., Zhang P., et al. . 2022. Inherited IFNAR1 deficiency in a child with both critical COVID-19 pneumonia and multisystem inflammatory syndrome. J. Clin. Immunol. 42:471–483. 10.1007/s10875-022-01215-7 - DOI - PMC - PubMed
-
- Acosta-Ampudia, Y., Monsalve D.M., Rojas M., Rodríguez Y., Gallo J.E., Salazar-Uribe J.C., Santander M.J., Cala M.P., Zapata W., Zapata M.I., et al. . 2021. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmun. 118:102598. 10.1016/j.jaut.2021.102598 - DOI - PMC - PubMed
-
- Akbil, B., Meyer T., Stubbemann P., Thibeault C., Staudacher O., Niemeyer D., Jansen J., Mühlemann B., Doehn J., Tabeling C., et al. . 2022. Early and rapid identification of COVID-19 patients with neutralizing type I interferon auto-antibodies. J. Clin. Immunol. 42:1111–1129. 10.1007/s10875-022-01252-2 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- EQU201903007798/French Foundation for Medical Research
- Meyer Foundation
- ANR-10-IAHU-01/Agence Nationale de la Recherche
- 1176665/National Health and Medical Research Council of Australia
- 08061821/San Matteo Hospital
- Imagine Institute
- R01 AI163029/AI/NIAID NIH HHS/United States
- Paris Cité University
- Rockefeller University
- Fondazione IRCCS Policlinico San Matteo
- UL1 TR001866/TR/NCATS NIH HHS/United States
- R01 AI124690/AI/NIAID NIH HHS/United States
- Battersea & Bowery Advisory Group
- R01AI163029/NH/NIH HHS/United States
- Grandir - Fonds de solidarité pour l'enfance
- Institut National de la Santé et de la Recherche Médicale
- P01 AI138938/AI/NIAID NIH HHS/United States
- RC08061819/Italian Ministry of Health
- French Ministry of Higher Education, Research, and Innovation
- HHMI/Howard Hughes Medical Institute/United States
- Square Foundation
- Fondation du Souffle
- SCOR Corporate Foundation for Science
- St. Giles Foundation
- 1176665/National Health and Medical Research Council
- R00 AI148604/AI/NIAID NIH HHS/United States
- Bettencourt-Schueller Foundation
- R21 AI142010/AI/NIAID NIH HHS/United States
- JPB Foundation
- ANRS0348/ANRS-MIE
- Stavros Niarchos Foundation
- Fisher Center for Alzheimer's Research Foundation
LinkOut - more resources
Full Text Sources

