Detailing organelle division and segregation in Plasmodium falciparum
- PMID: 39485315
- PMCID: PMC11535888
- DOI: 10.1083/jcb.202406064
Detailing organelle division and segregation in Plasmodium falciparum
Abstract
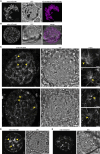
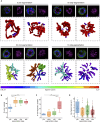
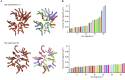
The malaria-causing parasite, P. falciparum, replicates through schizogony, a tightly orchestrated process where numerous daughter parasites are formed simultaneously. Proper division and segregation of one-per-cell organelles, like the mitochondrion and apicoplast, are essential, yet remain poorly understood. We developed a new reporter parasite line that allows visualization of the mitochondrion in blood and mosquito stages. Using high-resolution 3D imaging, we found that the mitochondrion orients in a cartwheel structure, prior to stepwise, non-geometric division during last-stage schizogony. Analysis of focused ion beam scanning electron microscopy data confirmed these mitochondrial division stages. Furthermore, these data allowed us to elucidate apicoplast division steps, highlighted its close association with the mitochondrion, and showed putative roles of the centriolar plaques in apicoplast segregation. These observations form the foundation for a new detailed mechanistic model of mitochondrial and apicoplast division and segregation during P. falciparum schizogony and pave the way for future studies into the proteins and protein complexes involved in organelle division and segregation.
© 2024 Verhoef et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures












Update of
-
Detailing organelle division and segregation in Plasmodium falciparum.bioRxiv [Preprint]. 2024 May 13:2024.01.30.577899. doi: 10.1101/2024.01.30.577899. bioRxiv. 2024. Update in: J Cell Biol. 2024 Dec 2;223(12):e202406064. doi: 10.1083/jcb.202406064. PMID: 38352445 Free PMC article. Updated. Preprint.
References
-
- Alvarez-Jarreta, J., Amos B., Aurrecoechea C., Bah S., Barba M., Barreto A., Basenko E.Y., Belnap R., Blevins A., Böhme U., et al. 2024. VEuPathDB: The eukaryotic pathogen, vector and host bioinformatics resource center in 2023. Nucleic Acids Res. 52:D808–D816. 10.1093/nar/gkad1003 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

