Pulmonary Atelectasis After Sedation With Propofol vs Propofol-Ketamine for Magnetic Resonance Imaging in Children: A Randomized Clinical Trial
- PMID: 39485355
- PMCID: PMC11530935
- DOI: 10.1001/jamanetworkopen.2024.33029
Pulmonary Atelectasis After Sedation With Propofol vs Propofol-Ketamine for Magnetic Resonance Imaging in Children: A Randomized Clinical Trial
Abstract
Importance: Little is known about the impact of different anesthetic agents used for routine magnetic resonance imaging (MRI) sedation on pulmonary function in children.
Objective: To compare the incidence of pulmonary atelectasis after MRI sedation with propofol vs propofol-ketamine.
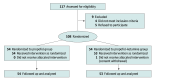
Design, setting, and participants: This double-masked randomized clinical trial screened 117 consecutive pediatric patients aged 3 to 12 years with American Society of Anesthesiologists physical status I to II undergoing elective MRI under deep sedation from November 2, 2022, to April 28, 2023, at a tertiary referral center. Four patients met the exclusion criteria, and 5 patients refused to participate. The participants and outcome assessors were masked to the group allocation.
Interventions: During the MRI, the propofol group received 0.2 mL/kg of 1% propofol and 2 mL of 0.9% saline followed by a continuous infusion of propofol (200 μg/kg/min) and 0.9% saline (0.04 mL/kg/min). The propofol-ketamine group received 0.2 mL/kg of 0.5% propofol and 1 mg/kg of ketamine followed by a continuous infusion of propofol (100 μg/kg/min) and ketamine (20 μg/kg/min).
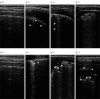
Main outcome and measure: The incidence of atelectasis assessed by lung ultrasonography examination.
Results: A total of 107 children (median [IQR] age, 5 [4-6] years; 62 male [57.9%]), with 54 in the propofol group and 53 in the propofol-ketamine group, were analyzed in this study. Notably, 48 (88.9%) and 31 (58.5%) patients had atelectasis in the propofol and propofol-ketamine groups, respectively (relative risk, 0.7; 95% CI, 0.5-0.8; P < .001). The incidence of desaturation and interruption of the MRI due to airway intervention or spontaneous movement did not significantly differ between the groups. The propofol-ketamine group showed a faster emergence time than the propofol group (15 [9-23] vs 25 [22-27] minutes in the propofol-ketamine vs propofol group; median difference in time, 9.0 minutes; 95% CI, 6.0-12.0 minutes; P < .001). No patient was withdrawn from the trial due to adverse effects.
Conclusions and relevance: In this randomized clinical trial, the propofol-ketamine combination reduced sedation-induced atelectasis while allowing for faster emergence compared with propofol alone.
Trial registration: cris.nih.go.kr Identifier: KCT0007699.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

