The Pharmacogenomics Global Research Network Implementation Working Group: global collaboration to advance pharmacogenetic implementation
- PMID: 39485373
- PMCID: PMC11664750
- DOI: 10.1097/FPC.0000000000000547
The Pharmacogenomics Global Research Network Implementation Working Group: global collaboration to advance pharmacogenetic implementation
Abstract
Pharmacogenetics promises to optimize treatment-related outcomes by informing optimal drug selection and dosing based on an individual's genotype in conjunction with other important clinical factors. Despite significant evidence of genetic associations with drug response, pharmacogenetic testing has not been widely implemented into clinical practice. Among the barriers to broad implementation are limited guidance for how to successfully integrate testing into clinical workflows and limited data on outcomes with pharmacogenetic implementation in clinical practice. The Pharmacogenomics Global Research Network Implementation Working Group seeks to engage institutions globally that have implemented pharmacogenetic testing into clinical practice or are in the process or planning stages of implementing testing to collectively disseminate data on implementation strategies, metrics, and health-related outcomes with the use of genotype-guided drug therapy to ultimately help advance pharmacogenetic implementation. This paper describes the goals, structure, and initial projects of the group in addition to implementation priorities across sites and future collaborative opportunities.
Copyright © 2024 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
J.N.P. is a consultant for VieCure and Clarified Precision Medicine. C.B. is founder of Sequence2Script Inc. J.R.B. is a consultant for OptumRx. N.K.T. is a member of the Association of Pathology Chairs (APC) Advocacy Working Group, Chair-elect of the Critical and Point-of-Care Testing Division, Association for Diagnostics and Laboratory Medicine (ADLM). J.D.A. is a consultant to Nurture Genomics and Precision Genetics. For the remaining authors, there are no conflicts of interest.
Figures
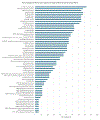
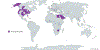


References
-
- The Pharmacogenomics Global Research Network. https://www.pgrn.org/. [Accessed 13 February 2024]
-
- Clinical Pharmacogenetics Implementation Consortium Guidelines. https://cpicpgx.org/guidelines/. [Accessed 14 May 2024]
-
- Swen JJ, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee AH, Mulder H, et al. Pharmacogenetics: from bench to byte—an update of guidelines. Clin Pharmacol Ther 2011; 89:662–673. - PubMed
-
- National Library of Medicine, National Center for Biotechnology Information. GTR: Genetic Testing Registry. https://www.ncbi.nlm.nih.gov/gtr/all/tests/?term=(testpurpose_drugrespon.... [Accessed 16 May 2024]

