Clinical Risk Factors for High-Dose Methotrexate-Induced Oral Mucositis Following Individualized Dosing
- PMID: 39485718
- PMCID: PMC11529650
- DOI: 10.1002/cam4.70351
Clinical Risk Factors for High-Dose Methotrexate-Induced Oral Mucositis Following Individualized Dosing
Abstract
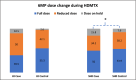
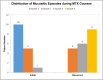
Background: Oral mucositis affects about 20% of children undergoing high-dose methotrexate (HDMTX) for acute lymphoblastic leukemia (ALL), despite existing management strategies. Personalized HDMTX dosing, adjusted by pharmacokinetics and leukemia risk, has reduced mucositis incidence, but variations still occur with similar 24-h methotrexate levels.
Methods: This retrospective study investigated risk factors for oral mucositis under individualized methotrexate protocols. Data from patients with ≥ Grade 2 oral mucositis (CTCAE v4.0) were analyzed from the St. Jude Children's Research Hospital total 16 trial. A 1:1 case-control matching method considered age, sex, risk classification, immunophenotype, and methotrexate course. McNemar's, Bowker's symmetry, and Wilcoxon signed-rank tests were used for statistical analyses. Risk factors for recurrent mucositis were identified in a case-only analysis.
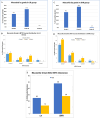
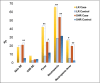
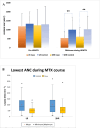
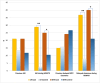
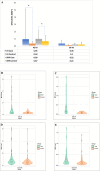
Results: The study found significant associations between methotrexate-induced mucositis and new-onset skin rashes (p = 0.0027), fever (p = 0.0016), neutropenic fever (p = 0.0008), lower absolute neutrophil count (p < 0.0001), acute kidney injury (AKI) (p = 0.0164), delayed methotrexate clearance (p = 0.0133), and higher 42-h methotrexate levels (p = 0.0179). In the standard/high-risk group, mercaptopurine dose was also linked to mucositis (p = 0.0495). Multivariable analysis showed that skin rashes (OR 6.5, p = 0.0016), fever (OR 2.8, p = 0.009), and neutropenia (OR 2.3, p = 0.0106) were independent risk factors for mucositis. Female sex (OR 7.12, p = 0.015) and AKI (OR 3.819, p = 0.037) were associated with recurrent mucositis.
Conclusions: Fever, skin rashes, AKI, delayed methotrexate clearance, and higher 42-h methotrexate levels were key risk factors for HDMTX-induced oral mucositis. Skin rashes, fever, and neutropenia were independent predictors, while female sex and AKI were linked to recurrent mucositis.
Keywords: acute kidney injury; acute lymphoblastic leukemia; delayed methotrexate clearance; high‐dose methotrexate; oral mucositis; risk factors.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
C.‐H.P. has received honoraria from Novartis, Amgen, and UpToDate and is on the Scientific Advisory board of Adaptive Inc. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Effectiveness of Parenteral Glutamine on Methotrexate-induced Oral Mucositis in Children with Acute Lymphoblastic Leukemia.Nutr Cancer. 2017 Jul;69(5):746-751. doi: 10.1080/01635581.2017.1324995. Epub 2017 Jun 1. Nutr Cancer. 2017. PMID: 28569624
-
Relationship between oral mucositis and high-dose methotrexate therapy in pediatric acute lymphoblastic leukemia.Int J Clin Pharmacol Ther. 2008 Nov;46(11):584-90. doi: 10.5414/cpp46584. Int J Clin Pharmacol Ther. 2008. PMID: 19000557 Clinical Trial.
-
Glutamine Mouthwash for Preventing Methotrexate-Induced Mucositis in Children with Acute Lymphoblastic Leukemia: A Randomized Cross-Over Trial.Indian Pediatr. 2025 Apr;62(4):269-275. doi: 10.1007/s13312-025-00042-4. Epub 2025 Apr 8. Indian Pediatr. 2025. PMID: 40198535 Clinical Trial.
-
Preventing and Managing Toxicities of High-Dose Methotrexate.Oncologist. 2016 Dec;21(12):1471-1482. doi: 10.1634/theoncologist.2015-0164. Epub 2016 Aug 5. Oncologist. 2016. PMID: 27496039 Free PMC article. Review.
-
The effect of leucovorin rescue therapy on methotrexate-induced oral mucositis in the treatment of paediatric ALL: A systematic review.Crit Rev Oncol Hematol. 2019 Oct;142:1-8. doi: 10.1016/j.critrevonc.2019.07.003. Epub 2019 Jul 9. Crit Rev Oncol Hematol. 2019. PMID: 31323533
Cited by
-
Mechanisms of Total Glucosides of Paeony in Alleviating Methotrexate-Induced Liver Injury.Drug Des Devel Ther. 2025 Apr 29;19:3407-3423. doi: 10.2147/DDDT.S521740. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40322039 Free PMC article.
References
-
- Ackland S. P. and Schilsky R. L., “High‐Dose Methotrexate: A Critical Reappraisal,” Journal of Clinical Oncology 5, no. 12 (1987): 2017–2031. - PubMed
-
- Abromowitch M., Ochs J., Pui C. H., et al., “High‐Dose Methotrexate Improves Clinical Outcome in Children With Acute Lymphoblastic Leukemia: St. Jude Total Therapy Study X,” Medical and Pediatric Oncology 16, no. 5 (1988): 297–303. - PubMed
-
- Abromowitch M., Ochs J., Pui C. H., Fairclough D., Murphy S. B., and Rivera G. K., “Efficacy of High‐Dose Methotrexate in Childhood Acute Lymphocytic Leukemia: Analysis by Contemporary Risk Classifications,” Blood 71, no. 4 (1988): 866–869. - PubMed
-
- Hill F. G., Richards S., Gibson B., et al., “Successful Treatment Without Cranial Radiotherapy of Children Receiving Intensified Chemotherapy for Acute Lymphoblastic Leukaemia: Results of the Risk‐Stratified Randomized Central Nervous System Treatment Trial MRC UKALL XI (ISRC TN 16757172),” British Journal of Haematology 124, no. 1 (2004): 33–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

