Proteomimetic polymer blocks mitochondrial damage, rescues Huntington's neurons, and slows onset of neuropathology in vivo
- PMID: 39485846
- PMCID: PMC11529722
- DOI: 10.1126/sciadv.ado8307
Proteomimetic polymer blocks mitochondrial damage, rescues Huntington's neurons, and slows onset of neuropathology in vivo
Abstract
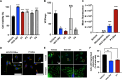
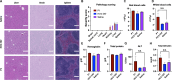
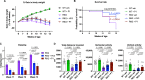
Recently, it has been shown that blocking the binding of valosin-containing protein (VCP) to mutant huntingtin (mtHtt) can prevent neuronal mitochondrial autophagy in Huntington's disease (HD) models. Herein, we describe the development and efficacy of a protein-like polymer (PLP) for inhibiting this interaction in cellular and in vivo models of HD. PLPs exhibit bioactivity in HD mouse striatal cells by successfully inhibiting mitochondrial destruction. PLP is notably resilient to in vitro enzyme, serum, and liver microsome stability assays, which render analogous control oligopeptides ineffective. PLP demonstrates a 2000-fold increase in circulation half-life compared to peptides, exhibiting an elimination half-life of 152 hours. In vivo efficacy studies in HD transgenic mice (R6/2) confirm the superior bioactivity of PLP compared to free peptide through behavioral and neuropathological analyses. PLP functions by preventing pathologic VCP/mtHtt binding in HD animal models; exhibits enhanced efficacy over the parent, free peptide; and implicates the PLP as a platform with potential for translational central nervous system therapeutics.
Figures
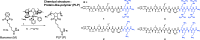

References
-
- Pan L., Feigin A., Huntington’s disease: New frontiers in therapeutics. Curr. Neurol. Neurosci. Rep. 21, 10 (2021). - PubMed
-
- Gupta S., Khan A., Vishwas S., Gulati M., Gurjeet T., Dua K., Kumar S., Najda A., Sayed A. A., Almeer R., Abdel-daim M. M., Demethyleneberberine: A possible treatment for Huntington’s disease. Med. Hypotheses 153, 110639 (2021). - PubMed
-
- Gibson J. S., Isaacs D. A., Claassen D. O., Stovall J. G., Lifetime neuropsychiatric symptoms in Huntington’s disease: Implications for psychiatric nursing. Arch. Psychiatr. Nurs. 35, 284–289 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

