Suppression of non-muscle myosin II boosts T cell cytotoxicity against tumors
- PMID: 39485850
- PMCID: PMC11529714
- DOI: 10.1126/sciadv.adp0631
Suppression of non-muscle myosin II boosts T cell cytotoxicity against tumors
Abstract
Increasing evidence highlights the importance of immune mechanoregulation in establishing and sustaining tumor-specific cytotoxicity required for desirable immunotherapeutic outcomes. However, the molecular connections between mechanobiological inputs and outputs and the designated immune activities remain largely unclear. Here, we show that partial inhibition of non-muscle myosin II (NM II) augmented the traction force exerted by T cells and potentiated T cell cytotoxicity against tumors. By using T cells from mice and patients with cancer, we found that NM II is required for the activity of NKX3-2 in maintaining the expression of ADGRB3, which shapes the filamentous actin (F-actin) organization and ultimately attributes to the reduced traction force of T cells in the tumor microenvironment. In animal models, suppressing the NM II-NKX3-2-ADGRB3 pathway in T cells effectively suppressed tumor growth and improved the efficacy of the checkpoint-specific immunotherapy. Overall, this work provides insights into the biomechanical regulation of T cell cytotoxicity that can be exploited to optimize clinical immunotherapies.
Figures
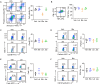

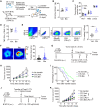

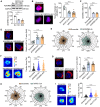


References
-
- Bareche Y., Kelly D., Abbas-Aghababazadeh F., Nakano M., Esfahani P. N., Tkachuk D., Mohammad H., Samstein R., Lee C. H., Morris L. G. T., Bedard P. L., Haibe-Kains B., Stagg J., Leveraging big data of immune checkpoint blockade response identifies novel potential targets. Ann. Oncol. 33, 1304–1317 (2022). - PubMed
-
- Lei K., Kurum A., Kaynak M., Bonati L., Han Y., Cencen V., Gao M., Xie Y. Q., Guo Y., Hannebelle M. T. M., Wu Y., Zhou G., Guo M., Fantner G. E., Sakar M. S., Tang L., Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy. Nat. Biomed. Eng. 5, 1411–1425 (2021). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

