Membrane potential stimulates ADP import and ATP export by the mitochondrial ADP/ATP carrier due to its positively charged binding site
- PMID: 39485853
- PMCID: PMC11529707
- DOI: 10.1126/sciadv.adp7725
Membrane potential stimulates ADP import and ATP export by the mitochondrial ADP/ATP carrier due to its positively charged binding site
Abstract
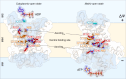
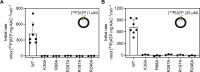
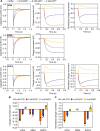
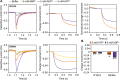
The mitochondrial adenosine 5'-diphosphate (ADP)/adenosine 5'-triphosphate (ATP) carrier imports ADP into the mitochondrion and exports ATP to the cell. Here, we demonstrate that 3.3 positive charges are translocated with the negatively charged substrate in each transport step. They can be assigned to three positively charged residues of the central substrate-binding site and two asparagine/arginine pairs. In this way, the membrane potential stimulates not only the ATP4- export step, as a net -0.7 charge is transported, but also the ADP3- import step, as a net +0.3 charge is transported with the electric field. These positive charge movements also inhibit the import of ATP and export of ADP in the presence of a membrane potential, allowing these nucleotides to be maintained at high concentrations in the cytosol and mitochondrial matrix to drive the hydrolysis and synthesis of ATP, respectively. Thus, this is the mechanism by which the membrane potential drives adenine nucleotide exchange with high directional fluxes to fuel the cellular processes.
Figures

References
-
- Ruprecht J. J., Kunji E. R. S., Structural mechanism of transport of mitochondrial carriers. Annu. Rev. Biochem. 90, 535–558 (2021). - PubMed
-
- Kunji E. R., Aleksandrova A., King M. S., Majd H., Ashton V. L., Cerson E., Springett R., Kibalchenko M., Tavoulari S., Crichton P. G., Ruprecht J. J., The transport mechanism of the mitochondrial ADP/ATP carrier. Biochim. Biophys. Acta 1863, 2379–2393 (2016). - PubMed
-
- R. Garrett, C. M. Grisham, Biochemistry (Brooks/Cole, Cengage Learning, ed. 4, 2010).
-
- Buono M. J., Kolkhorst F. W., Estimating ATP resynthesis during a marathon run: A method to introduce metabolism. Adv. Physiol. Educ. 25, 70–71 (2001).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

