Neonatal hypoxia-ischemia alters the events governing the hippocampal critical period of postnatal synaptic plasticity leading to deficits in working memory in mice
- PMID: 39486775
- PMCID: PMC11646096
- DOI: 10.1016/j.nbd.2024.106722
Neonatal hypoxia-ischemia alters the events governing the hippocampal critical period of postnatal synaptic plasticity leading to deficits in working memory in mice
Abstract
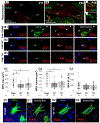
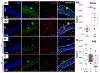
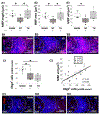
Postnatal critical periods of synaptic plasticity (CPsp) are characterized by profound neural network refinement, which is shaped by synaptic activity and sculpted by maturation of the GABAergic network. Even after therapeutic hypothermia (TH), neonatal hypoxia-ischemia (HI) impairs two triggers for the initiation of the CPsp in the hippocampus: i) PSA-NCAM developmental decline and ii) parvalbumin (PV) + interneuron (IN) maturation. Thus, we investigated whether neonatal HI despite TH disturbs other events governing the onset, consolidation and closure of the postnatal CPsp in the hippocampus. We induced cerebral HI in P10 C57BL6 mice with right carotid ligation and 45 m of hypoxia (FiO2 = 0.08), followed by normothermia (36 °C, NT) or TH (31 °C) for 4 h with anesthesia-exposed shams as controls. ELISA, immunoblotting and immunohistochemistry were performed at 24 h (P11), 5 days (P15), 8 days (P18) and 30 days (P40) after HI injury. We specifically assessed: i) BDNF levels and TrkB activation, controlling the CPsp, ii) Otx2 and NPTX2 immunoreactivity (IR), engaging CPsp onset and iii) NogoR1, Lynx1 IR, PNN formation and myelination (MBP) mediating CPsp closure. Pups aged to P40 also received a battery of tests assessing working memory. Here, we documented deficits in hippocampal BDNF levels and TrkB activation at P18 in response to neonatal HI even with TH. Neonatal HI impaired in the CA1 the developmental increase in PV, Otx2, and NPTX2 between P11 and P18, the colocalization of Otx2 and PV at P18 and P40, the accumulation of NPTX2 in PV+ dendrites at P18 and P40, and the expression of NogoR at P40. Furthermore, neonatal HI decreased BDNF and impaired PNN development and myelination (MBP) at P40. Most of these abnormalities were insensitive to TH and correlated with memory deficits. Neonatal HI appears to disrupt many of the molecular and structural events initiating and consolidating the postnatal hippocampal CPsp, perhaps due to the early and delayed deficits in TrkB activation leading to memory deficits.
Keywords: CA1; Interneurons; Memory; Neonatal hypoxia-ischemia; Parvalbumin; Synaptic plasticity; hippocampus.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Raul Chavez-Valdez reports financial support was provided by National Institutes of Health. Raul Chavez-Valdez reports financial support was provided by Thomas Wilson Sanitarium for Children of Baltimore City. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Azzopardi D, et al. , 2014. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med 371, 140–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

