Living systematic review and comprehensive network meta-analysis of ALS clinical trials: study protocol
- PMID: 39486809
- PMCID: PMC11529510
- DOI: 10.1136/bmjopen-2024-087970
Living systematic review and comprehensive network meta-analysis of ALS clinical trials: study protocol
Abstract
Introduction: Amyotrophic lateral sclerosis (ALS) is a fatal neurogenerative disease with no effective treatment to date. Despite numerous clinical trials, the majority of studies have been futile in their effort to significantly alter the course of the disease. However, these studies may still provide valuable information for identifying patient subgroups and generating new hypotheses for future research. Additionally, synthesising evidence from these studies may help overcome the limitations of individual studies. Network meta-analysis may refine the assessment of efficacy in specific patient subgroups, evaluate intervention characteristics such as mode of administration or biological mechanisms of action, and rank order promising therapeutic areas of interest. Therefore, we aim to synthesise the available evidence from ALS clinical trials.
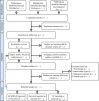
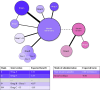
Methods and analysis: We will conduct a systematic review to identify all clinical trials that assessed disease-modifying pharmaceutical therapies, cell therapies, or supplements in patients with ALS. Outcomes of interest are clinical disease progression outcomes and survival. We will conduct this search in the period Q4 2024 in three databases: PubMed, Embase and ClinicalTrials.gov for studies from 1999 to 2023. Individual patient data and aggregate data will be collected and subsequentially synthesised in meta-analytical models. The final model will be presented as an open-source web application with biannual updates of the underlying data, thereby providing a 'living' overview of the ALS clinical trial landscape.
Ethics and dissemination: No ethics approvals are required. Findings will be presented at relevant conferences and submitted to peer-reviewed journals. Data will be stored anonymously in secure repositories.
Keywords: network meta-analysis; neuromuscular disease; systematic review.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
