Insights into the Hierarchical Assembly of a Chemically Diverse Peptide Hydrogel Derived from Human Semenogelin I
- PMID: 39487039
- PMCID: PMC11562788
- DOI: 10.1021/acsnano.4c08672
Insights into the Hierarchical Assembly of a Chemically Diverse Peptide Hydrogel Derived from Human Semenogelin I
Abstract
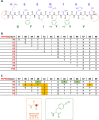
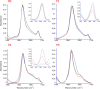
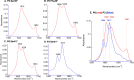
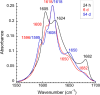
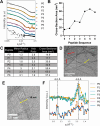
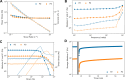
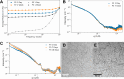
A peptide corresponding to a 13-residue segment of the human protein semenogelin I has been shown to generate a hydrogel consisting of amyloid-like fibrils. The relative chemical diversity (compared to synthetic de novo sequences) with 11 distinct amino acids makes this peptide (P0) an ideal candidate for investigating the role of individual residues in gelation. Herein, the N-terminal residues have been sequentially removed to furnish a series of truncated peptides, P1-P10, ranging from 12 to 3 residues in length. FTIR spectroscopy investigations reveal that P0-P6 forms a β-sheet secondary structure while shorter sequences do not self-assemble. Site-specific isotope labeling of the amide backbone of P0-P2 with the IR-sensitive vibrational probe 13C═O yields FTIR spectra indicative of the initial formation of a kinetic product that slowly transforms into a structurally different thermodynamic product. The effects of the isotopic labels on the IR spectra facilitate the assignment of parallel and antiparallel structures, which are sometimes coexistent. Additional IR studies of three PheCN-labeled P0 sequences are consistent with an H-bonded β-sheet amide core, spanning the 7 central residues. The macromolecular assembly of peptides that form β-sheets was assessed by cryo-TEM, SAXS/WAXS, and rheology. Cryo-TEM images of peptides P1-P6 display μm-long nanofibrils. Peptides P0-P3 generate homogeneous hydrogels composed of colloidally stable nanofibrils, and P4-P6 undergo phase separation due to the accumulation of attractive interfibrillar interactions. Three amino acid residues, Ser39, Phe40, and Gln43, were identified to be of particular interest in the truncated peptide series as the removal of any one of them, as the sequence shortens, leads to a major change in material properties.
Keywords: X-ray scattering; amyloid; isotope effects; peptide aggregation; protein fragment; self-assembly; supramolecular assembly.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Jonker A. M.; Löwik D. W. P. M.; van Hest J. C. M. Peptide- and Protein-Based Hydrogels. Chem. Mater. 2012, 24 (5), 759–773. 10.1021/cm202640w. - DOI
-
- Zhao W.; Jin X.; Cong Y.; Liu Y.; Fu J. Degradable Natural Polymer Hydrogels for Articular Cartilage Tissue Engineering. J. Chem. Technol. Biotechnol. 2013, 88 (3), 327–339. 10.1002/jctb.3970. - DOI
-
- Dasgupta A.; Mondal J. H.; Das D. Peptide Hydrogels. RSC Adv. 2013, 3 (24), 9117–9149. 10.1039/c3ra40234g. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

