Increased DNA damage in full-grown oocytes is correlated with diminished autophagy activation
- PMID: 39487138
- PMCID: PMC11530536
- DOI: 10.1038/s41467-024-53559-w
Increased DNA damage in full-grown oocytes is correlated with diminished autophagy activation
Abstract
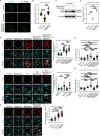
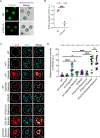
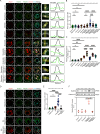
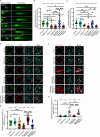
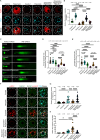
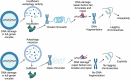
Unlike mild DNA damage exposure, DNA damage repair (DDR) is reported to be ineffective in full-grown mammalian oocytes exposed to moderate or severe DNA damage. The underlying mechanisms of this weakened DDR are unknown. Here, we show that moderate DNA damage in full-grown oocytes leads to aneuploidy. Our data reveal that DNA-damaged oocytes have an altered, closed, chromatin state, and suggest that the failure to repair damaged DNA could be due to the inability of DDR proteins to access damaged loci. Our data also demonstrate that, unlike somatic cells, mouse and porcine oocytes fail to activate autophagy in response to DNA double-strand break-inducing treatment, which we suggest may be the cause of the altered chromatin conformation and inefficient DDR. Importantly, autophagy activity is further reduced in maternally aged oocytes (which harbor severe DNA damage), and its induction is correlated with reduced DNA damage in maternally aged oocytes. Our findings provide evidence that reduced autophagy activation contributes to weakened DDR in oocytes, especially in those from aged females, offering new possibilities to improve assisted reproductive therapy in women with compromised oocyte quality.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Centers for Disease, C. & Prevention. (2019).
-
- American College of, O. & Gynecologists. (2020).
-
- Hassold, T., Hall, H. & Hunt, P. The origin of human aneuploidy: where we have been, where we are going. Hum. Mol. Genet.16, R203–R208 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

