A DNA base-specific sequence interposed between CRX and NRL contributes to RHODOPSIN expression
- PMID: 39487168
- PMCID: PMC11530525
- DOI: 10.1038/s41598-024-76664-8
A DNA base-specific sequence interposed between CRX and NRL contributes to RHODOPSIN expression
Abstract
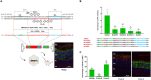
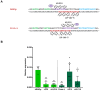
Gene expression emerges from DNA sequences through the interaction of transcription factors (TFs) with DNA cis-regulatory sequences. In eukaryotes, TFs bind to transcription factor binding sites (TFBSs) with differential affinities, enabling cell-specific gene expression. In this view, DNA enables TF binding along a continuum ranging from low to high affinity depending on its sequence composition; however, it is not known whether evolution has entailed a further level of entanglement between DNA-protein interaction. Here we found that the composition and length (22 bp) of the DNA sequence interposed between the CRX and NRL retinal TFs in the proximal promoter of RHODOPSIN (RHO) largely controls the expression levels of RHO. Mutagenesis of CRX-NRL DNA linking sequences (here termed "DNA-linker") results in uncorrelated gene expression variation. In contrast, mutual exchange of naturally occurring divergent human and mouse Rho cis-regulatory elements conferred similar yet species-specific Rho expression levels. Two orthogonal DNA-binding proteins targeted to the DNA-linker either activate or repress the expression of Rho depending on the DNA-linker orientation relative to the CRX and NRL binding sites. These results argue that, in this instance, DNA itself contributes to CRX and NRL activities through a code based on specific base sequences of a defined length, ultimately determining optimal RHO expression levels.
© 2024. The Author(s).
Conflict of interest statement
E.M.S., S.B. and E.M. are inventors on patents, “Artificial DNA-binding proteins and uses thereof”, WO2015075154A3, US20160289284A1. E.M.S., S.B. and E.M. are inventors on a pending patent “Ectopically expressed transcription factors and uses thereof”, PCT/EP2018/086782, Department of Translational Medical Sciences (DiSMeT) University of Naples "Federico II", Italy, which may encompass the findings. The remaining authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

