Investigation in the cannabigerol derivative VCE-003.2 as a disease-modifying agent in a mouse model of experimental synucleinopathy
- PMID: 39487447
- PMCID: PMC11531178
- DOI: 10.1186/s12993-024-00256-9
Investigation in the cannabigerol derivative VCE-003.2 as a disease-modifying agent in a mouse model of experimental synucleinopathy
Abstract
Background: The cannabigerol derivative VCE-003.2, which has activity at the peroxisome proliferator-activated receptor-γ has afforded neuroprotection in experimental models of Parkinson's disease (PD) based on mitochondrial dysfunction (6-hydroxydopamine-lesioned mice) and neuroinflammation (LPS-lesioned mice). Now, we aim to explore VCE-003.2 neuroprotective properties in a PD model that also involves protein dysregulation, other key event in PD pathogenesis.
Methods: To this end, an adeno-associated viral vector serotype 9 coding for a mutated form of the α-synuclein gene (AAV9-SynA53T) was unilaterally delivered in the substantia nigra pars compacta (SNpc) of mice. This model leads to motor impairment and progressive loss of tyrosine hydroxylase-labelled neurons in the SNpc.
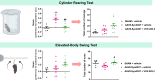
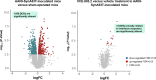
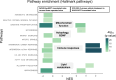
Results: Oral administration of VCE-003.2 at 20 mg/kg for 14 days improved the performance of mice injected with AAV9-SynA53T in various motor tests, correlating with the preservation of tyrosine hydroxylase-labelled neurons in the SNpc. VCE-003.2 also reduced reactive microgliosis and astrogliosis in the SNpc. Furthermore, we conducted a transcriptomic analysis in the striatum of mice injected with AAV9-SynA53T and treated with either VCE-003.2 or vehicle, as well as control animals. This analysis aimed to identify gene families specifically altered by the pathology and/or VCE-003.2 treatment. Our data revealed pathology-induced changes in genes related to mitochondrial function, lysosomal cell pathways, immune responses, and lipid metabolism. In contrast, VCE-003.2 treatment predominantly affected the immune response through interferon signaling.
Conclusion: Our study broadens the neuroprotective potential of VCE-003.2, previously described against mitochondrial dysfunction, oxidative stress, glial reactivity and neuroinflammation in PD. We now demonstrate its efficacy against another key pathogenic event in PD as α-synuclein dysregulation. Furthermore, our investigation sheds light on the molecular mechanisms underlying VCE-003.2 revealing its role in regulating interferon signaling. These findings, together with a favorable ADMET profile, enhance the preclinical interest of VCE-003.2 towards its future clinical development in PD.
Keywords: Cannabinoids; PPAR-γ; Parkinson’s disease; VCE-003.2; alpha-synuclein.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Aguareles J, Paraíso-Luna J, Palomares B, Bajo-Grañeras R, Navarrete C, Ruiz-Calvo A, García-Rincón D, García-Taboada E, Guzmán M, Muñoz E, Galve-Roperh I. Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-induced neurodegeneration. Translational Neurodegeneration. 2019;8:9. - PMC - PubMed
-
- Anand S, Azam Ansari M, Kumaraswamy Sukrutha S, Alomary MN, Anwar Khan A, Elderdery AY. Resolvins lipid mediators: potential therapeutic targets in Alzheimer and Parkinson disease. Neuroscience. 2022;507:139–48. - PubMed
-
- Antonazzo M, Botta M, Bengoetxea H, Ruiz-Ortega JA, Morera-Herreras T. Therapeutic potential of cannabinoids as neuroprotective agents for damaged cells conducing to movement disorders. Int Rev Neurobiol. 2019;146:229–57. - PubMed
-
- Aymerich MS, Aso E, Abellanas MA, Tolon RM, Ramos JA, Ferrer I, Romero J, Fernández-Ruiz J. Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem Pharmacol. 2018;157:67–84. - PubMed
-
- Basurco L, Abellanas MA, Ayerra L, Conde E, Vinueza-Gavilanes R, Luquin E, Vales A, Vilas A, Martin-Uriz PS, Tamayo I, Alonso MM, Hernaez M, Gonzalez-Aseguinolaza G, Clavero P, Mengual E, Arrasate M, Hervás-Stubbs S, Aymerich MS. Microglia and astrocyte activation is region-dependent in the α-synuclein mouse model of Parkinson’s disease. Glia. 2022;71:571–87. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

