A scoping review, novel taxonomy and catalogue of implementation frameworks for clinical decision support systems
- PMID: 39487462
- PMCID: PMC11531160
- DOI: 10.1186/s12911-024-02739-1
A scoping review, novel taxonomy and catalogue of implementation frameworks for clinical decision support systems
Abstract
Background: The primary aim of this scoping review was to synthesise key domains and sub-domains described in existing clinical decision support systems (CDSS) implementation frameworks into a novel taxonomy and demonstrate most-studied and least-studied areas. Secondary objectives were to evaluate the frequency and manner of use of each framework, and catalogue frameworks by implementation stage.
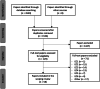
Methods: A scoping review of Pubmed, Scopus, Web of Science, PsychInfo and Embase was conducted on 12/01/2022, limited to English language, including 2000-2021. Each framework was categorised as addressing one or multiple stages of implementation: design and development, evaluation, acceptance and integration, and adoption and maintenance. Key parts of each framework were grouped into domains and sub-domains.
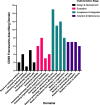
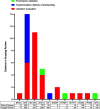
Results: Of 3550 titles identified, 58 papers were included. The most-studied implementation stage was acceptance and integration, while the least-studied was design and development. The three main framework uses were: for evaluating adoption, for understanding attitudes toward implementation, and for framework validation. The most frequently used framework was the Consolidated Framework for Implementation Research.
Conclusions: Many frameworks have been published to overcome barriers to CDSS implementation and offer guidance towards successful adoption. However, for co-developers, choosing relevant frameworks may be a challenge. A taxonomy of domains addressed by CDSS implementation frameworks is provided, as well as a description of their use, and a catalogue of frameworks listed by the implementation stages they address. Future work should ensure best practices for CDSS design are adequately described, and existing frameworks are well-validated. An emphasis on collaboration between clinician and non-clinician affected parties may help advance the field.
Keywords: Adoption; Clinical decision support system; Design; Development; Evaluation; Frameworks; Implementation; Scoping review.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Haynes RB, Wilczynski NL, the Computerized Clinical Decision Support System (CCDSS) Systematic Review Team. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: ethods of a decision-maker- researcher partnership systematic review. Implement Sci. 2010;5(12):1–8. - PMC - PubMed
-
- Adams ST, Leveson SH. Clinical prediction rules. BMJ. 2012;344: d8312. - PubMed
-
- Shortliffe EH, Cimino JJ. Biomedical Informatics: Computer Applications in Health Care and Biomedicine. New York: Springer; 2006. p. 1037.
-
- Medicine, IO. Leadership commitments to improve value in health care: finding common ground: Workshop summary. Washington. DC: National Academies Press; 2009. - PubMed
-
- Tcheng JE, Bakken S, Bates DW, Bonner H III, Gandhi TK, Josephs M, Kawamoto K, Lomotan EA, Mackay E, Middleton B, Teich JM, Weingarten S, Hamilton Lopez M, editors. Optimizing strategies for clinical decision support: summary of a meeting series. Washington, DC: National Academy of Medicine; 2017. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

