The role of alemtuzumab in the development of secondary autoimmunity in multiple Sclerosis: a systematic review
- PMID: 39487492
- PMCID: PMC11528992
- DOI: 10.1186/s12974-024-03263-9
The role of alemtuzumab in the development of secondary autoimmunity in multiple Sclerosis: a systematic review
Abstract
Background: Secondary autoimmune disease (SAID) in the context of alemtuzumab treatment is one of the main safety concerns that may arise following administration in people with multiple sclerosis (pwMS). Contributing factors underlying this adverse event are not well understood. The purpose of this systematic review was to appraise the literature investigating the role of alemtuzumab in the development of SAID in pwMS following treatment and identify potential biomarkers/ risk factors that may be predictive of onset of this manifestation.
Methods: Relevant publications were retrieved from PubMed, Embase, and Web of Science using a three-pronged search strategy containing the following keywords: "multiple sclerosis"; "alemtuzumab"; and "autoimmunity". Studies that fulfilled the specified eligibility criteria and investigated SAID development after alemtuzumab in pwMS were included in the final analysis.
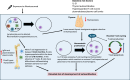
Results: 19 papers were included in the final review. Approximately, 47.92% of pwMS treated with alemtuzumab experienced SAID. A variety of biomarkers and risk factors were noted in the development of SAID, with a focus on immunological changes, including: increased homeostatic proliferation and T cell cycling, along with consistently elevated baseline serum IL-21 levels and thyroid autoantibodies. There was no significant association between known human leukocyte antigen (HLA) risk alleles, lymphocyte profile or dynamics and SAID development.
Conclusions: While the mechanism underlying SAID following alemtuzumab is not fully understood, potential biomarkers and risk factors that may assist in elucidating mechanisms underlying this phenomenon have been documented in several independent studies. Following immunodepletion from alemtuzumab, an IL-21 driven increase in homeostatic proliferation and T cell cycling may disrupt tolerance mechanisms leading to an increase in the propensity toward alemtuzumab-induced autoimmunity. Further research is necessary to clarify the physiological changes after alemtuzumab therapy that trigger SAID in pwMS.
Keywords: Alemtuzumab; Multiple Sclerosis; Secondary autoimmune disease.
© 2024. The Author(s).
Conflict of interest statement
The authors wish to declare that SJS and SAB are investigators on the RAMBLE trial (ACTRN12621001502820). The RAMBLE study has been funded by MS Australia (Project Grant 21-2-060). SAB has received support through Griffith University in relation to service on advisory boards, conference organising committees and speakers honoraria from Novartis, Roche and Alexion, and has been a principal investigator on clinical trials sponsored by ATARA, Biogen-Idec, Sanofi and UCB. RM, NEF, and SMG declare no conflicts of interest.
Figures

References
-
- Jakimovski D, et al. Multiple sclerosis. Lancet. 2024;403(10422):183–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

