Senolytic treatment diminishes microglia and decreases severity of experimental autoimmune encephalomyelitis
- PMID: 39487537
- PMCID: PMC11529445
- DOI: 10.1186/s12974-024-03278-2
Senolytic treatment diminishes microglia and decreases severity of experimental autoimmune encephalomyelitis
Abstract
Background: The role of senescence in disease contexts is complex, however there is considerable evidence that depletion of senescent cells improves outcomes in a variety of contexts particularly related to aging, cognition, and neurodegeneration. Much research has shown previously that inflammation can promote cellular senescence. Microglia are a central nervous system innate immune cell that undergo senescence with aging and during neurodegeneration. The contribution of senescent microglia to multiple sclerosis, an inflammatory neurodegenerative disease, is not clear, but microglia are strongly implicated in chronic active lesion pathology, tissue injury, and disease progression. Drugs that could specifically eliminate dysregulated microglia in multiple sclerosis are therefore of great interest to the field.
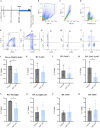
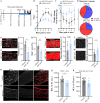
Results: A single-cell analysis of brain tissue from mice subjected to experimental autoimmune encephalomyelitis (EAE), a mouse model of CNS inflammation that models aspects of multiple sclerosis (MS), identified microglia with a strong transcriptional signature of senescence including the presence of BCL2-family gene transcripts. Microglia expressing Bcl2l1 had higher expression of pro-inflammatory and senescence associated genes than their Bcl2l1 negative counterparts in EAE, suggesting they may exacerbate inflammation. Notably, in human single-nucleus sequencing from MS, BCL2L1 positive microglia were enriched in lesions with active inflammatory pathology, and likewise demonstrated increased expression of immune genes suggesting they may be proinflammatory and contribute to disease processes in chronic active lesions. Employing a small molecule BCL2-family inhibitor, Navitoclax (ABT-263), significantly reduced the presence of microglia and macrophages in the EAE spinal cord, suggesting that these cells can be targeted by senolytic treatment. ABT-263 treatment had a profound effect on EAE mice: decreasing motor symptom severity, improving visual acuity, promoting neuronal survival, and decreasing white matter inflammation.
Conclusion: These results support the hypothesis that microglia and macrophages exhibit transcriptional features of cellular senescence in EAE and MS, and that microglia expressing Bcl2l1 demonstrate a proinflammatory signature that may exacerbate inflammation resulting in negative outcomes in neuroinflammatory disease. Depleting microglia and macrophages using a senolytic results in robust improvement in EAE disease severity, including across measures of neurodegeneration, inflammation, and demyelination, and may therefore represent a novel strategy to address disease progression in multiple sclerosis.
Keywords: Experimental autoimmune encephalomyelitis; Microglia; Multiple sclerosis; Neuroinflammation; Senescence; Senolytics; Senotherapeutic.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Filippi M, et al. Multiple sclerosis. Nat Reviews: Disease Primers. 2018;4. 10.1038/s41572-018-0041-4. - PubMed
-
- Drake SS, Zaman A, Simas T, Fournier AE. Comparing RNA-sequencing datasets from astrocytes, oligodendrocytes, and microglia in multiple sclerosis identifies novel dysregulated genes relevant to inflammation and myelination. WIREs Mech Dis. 2023;15:e1594. 10.1002/wsbm.1594. - PubMed
-
- Yong VW. Microglia in multiple sclerosis: protectors turn destroyers. Neuron. 2022;110:3534–48. 10.1016/j.neuron.2022.06.023. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

