The impact of post-remission granulocyte colony-stimulating factor use in the phase 3 studies of venetoclax combination treatments in patients with newly diagnosed acute myeloid leukemia
- PMID: 39487568
- PMCID: PMC11625987
- DOI: 10.1002/ajh.27515
The impact of post-remission granulocyte colony-stimulating factor use in the phase 3 studies of venetoclax combination treatments in patients with newly diagnosed acute myeloid leukemia
Conflict of interest statement
C. D. DiNardo: Research support to institution from AbbVie, Agios, Astex, Beigene, Bristol Myers Squibb, Cleave, Foghorn, Forma/Rigel, ImmuneOnc, Loxo, Servier; consultancy/advisory role with AbbVie, Astellas, Bristol Myers Squibb, Genentech, GenMab, Gilead Sciences, GlaxoSmithKline, Immunogen, Jazz, Novartis, Notable Labs, Rigel, Servier; supported by the LLS Scholar in Clinical Research Award. K. W. Pratz: Research funding from AbbVie, Agios, Daiichi Sankyo, Millennium; advisory board member for AbbVie, Astellas, AstraZeneca, Boston Biomedical, Bristol Myers Squibb, Celgene, Novartis, Roche, Jazz Pharmaceuticals, and Servier. P. Panayiotidis: Grants/research support from AbbVie, Roche, Novartis, and Genesis and honoraria from AbbVie, Janssen, Roche, Novartis, Gilead, and Genesis. X. Wei: No conflicts of interest to disclose. V. Vorobyev: Advisor/consultancy for AbbVie, Janssen, Celgene, Novartis, Pfizer, Takeda, Roche, BeiGene, Amgen, and Sanofi. Á. Illés: Advisor/consultancy for AbbVie, Janssen, Celgene, Novartis, Pfizer, Takeda, Roche. I. Kim: Investigator in AbbVie‐sponsored clinical trials. V. Ivanov: Investigator in AbbVie‐sponsored clinical trials, Novartis, Astellas, MSD, Biocad, and Ascentage Pharma‐sponsored clinical trials; and educational events for AbbVie, Pfizer, Novartis, Roche, Biocad, and Sotex. G. Ku: Employment with Genentech, Inc. and may hold stock or other options. C. L. Miller, M. Zhang, F. Tatsch, J. Potluri, and X. Schmidt: Employment with AbbVie and may hold stock or other options. C. Récher: Consulting or advisory role with AbbVie, Amgen, Astellas, Bristol Myers Squibb, Boehringer, Daiichi‐Sankyo, Jazz Pharmaceuticals, Janssen, and Servier; research funding from AbbVie, Amgen, Astellas, Bristol Myers Squibb, Daiichi‐Sankyo, Iqvia, and Jazz Pharmaceuticals; and support for attending meetings and/or travel from AbbVie, Novartis, and Servier.
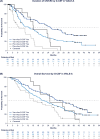
Figures
References
-
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617‐629. - PubMed
-
- Pratz KW, Jonas BA, Pullarkat V, et al. Long‐term follow‐up of VIALE‐A: Venetoclax and azacitidine in chemotherapy‐ineligible untreated acute myeloid leukemia. Am J Hematol. 2024;99:615‐624. - PubMed
-
- Wei AH, Panayiotidis P, Montesinos P, et al. Long‐term follow‐up of VIALE‐C in patients with untreated AML ineligible for intensive chemotherapy. Blood. 2022;140(25):2754‐2756. - PubMed
-
- Dohner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345‐1377. - PubMed
-
- Pratz KW, DiNardo CD, Selleslag D, et al. Postremission cytopenia management in patients with acute myeloid leukemia treated with venetoclax and azacitidine in VIALE‐A. Am J Hematol. 2022;97(11):E416‐E419. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
