A Phase 1 Study of ABI-009 (Nab-sirolimus) in Combination With Temozolomide and Irinotecan in Pediatric Patients With Recurrent or Refractory Solid Tumors, Including CNS Tumors-A Children's Oncology Group Pediatric Early Phase Clinical Trial Network Study ADVL1514
- PMID: 39487711
- PMCID: PMC11533328
- DOI: 10.1002/cam4.70376
A Phase 1 Study of ABI-009 (Nab-sirolimus) in Combination With Temozolomide and Irinotecan in Pediatric Patients With Recurrent or Refractory Solid Tumors, Including CNS Tumors-A Children's Oncology Group Pediatric Early Phase Clinical Trial Network Study ADVL1514
Abstract
Background: Nab-sirolimus (ABI-009, nab-rapamycin; Aadi Bioscience Inc. [Aadi]) is a human albumin-bound form of sirolimus nanoparticles, a potent mTOR inhibitor. This phase I trial was conducted to define dose-limiting toxicities (DLT), maximum tolerated or recommended phase II dose (MTD/RP2D), and pharmacokinetics of Nab-sirolimus in combination with temozolomide and irinotecan.
Methods: Using a rolling 6 design, Nab-sirolimus was administered intravenously (IV) on days (D) 1 and 8 of cycle (C) 1. In subsequent cycles, Nab-sirolimus was administered D1 and D8 in combination with temozolomide (125 mg/m2/dose, maximum 250 mg/dose) and irinotecan (90 mg/m2/dose) orally, daily on D1-5. Cycle duration was 21 days. Three dose levels (DL) of Nab-sirolimus were investigated (DL1: 35 mg/m2/dose, DL-1: 20 mg/m2/dose, and DL-2: 15 mg/m2/dose). The observation period for estimating the MTD/RP2D was defined by cycles 1 and 2.
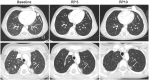
Results: Thirty-three patients were enrolled, 32 were eligible. Dose determination included 17 evaluable patients, median (range) age 12 (2-20) years and six additional patients were enrolled (four evaluable for toxicity) on a pharmacokinetic cohort. C1 or C2 DLTs were primarily thrombocytopenia including 2/5 patients at DL1, 2/6 patients at DL-1, and 1/6 patients at DL-2. One patient (DL1) with Ewing Sarcoma had a partial response and remained on study for 35 cycles. Rapamycin clearance was dose dependent. Irinotecan clearance and its active metabolite SN-38 exposure were not affected by coadministration with Nab-sirolimus.
Conclusion: The MTD for Nab-sirolimus was 15 mg/m2/dose IV on D1 and D8 in combination with temozolomide 125 mg/m2/dose and oral irinotecan 90 mg/m2/dose daily for 5 days during 21D cycles.
Trial registration: ClinicalTrials.gov identifier NCT02975882.
Keywords: ABI‐009; Clinical trial; Pediatrics; nab‐rapamycin; rapamycin.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Reid serves as a consultant for Elucida Oncology Inc.
The authors declare no conflicts of interest.
Figures

References
-
- Bagatell R., Norris R., Ingle A. M., et al., “Phase 1 Trial of Temsirolimus in Combination With Irinotecan and Temozolomide in Children, Adolescents and Young Adults With Relapsed or Refractory Solid Tumors: A Children's Oncology Group Study,” Pediatric Blood & Cancer 61, no. 5 (2014): 833–839. - PMC - PubMed
-
- Faivre S., Kroemer G., and Raymond E., “Current Development of mTOR Inhibitors as Anticancer Agents,” Nature Reviews. Drug Discovery 5, no. 8 (2006): 671–688. - PubMed
-
- Qiu J., Feng M., Yang G., et al., “mTOR Inhibitor, Gemcitabine and PD‐L1 Antibody Blockade Combination Therapy Suppresses Pancreatic Cancer Progression via Metabolic Reprogramming and Immune Microenvironment Remodeling in Trp53(Flox/+)LSL‐Kras(G12D/+)Pdx‐1‐Cre Murine Models,” Cancer Letters 554 (2023): 216020. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

