Older Adults Benefit From Virtual Support for Continuous Glucose Monitor Use But Require Longer Visits
- PMID: 39487727
- PMCID: PMC11571625
- DOI: 10.1177/19322968241294250
Older Adults Benefit From Virtual Support for Continuous Glucose Monitor Use But Require Longer Visits
Abstract
Background: Older adults may be less comfortable with continuous glucose monitoring (CGM) technology or require additional education to support use. The Virtual Diabetes Specialty Clinic study provided the opportunity to understand glycemic outcomes and support needed for older versus younger adults living with diabetes and using CGM.
Methods: Prospective, virtual study of adults with type 1 diabetes (T1D, N = 160) or type 2 diabetes (T2D, N = 74) using basal-bolus insulin injections or insulin pump therapy. Remote CGM diabetes education (3 scheduled visits over 1 month) was provided by Certified Diabetes Care and Education Specialists with additional visits as needed. CGM-measured glycemic metrics, HbA1c and visit duration were evaluated by age (<40, 40-64 and ≥65 years).
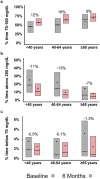
Results: Median CGM use was ≥95% in all age groups. From baseline to 6 months, time 70 to 180 mg/dL improved from 45% ± 22 to 57% ± 16%; 50 ± 25 to 65 ± 18%; and 60 ± 28 to 69% ± 18% in the <40, 40-64, and ≥65-year groups, respectively (<40 vs 40-64 years P = 0.006). Corresponding values for HbA1c were 8.0% ± 1.6 to 7.3% ± 1.0%; 7.9 ± 1.6 to 7.0 ± 1.0%; and 7.4 ± 1.4 to 7.1% ± 0.9% (all P > 0.05). Visit duration was 41 min longer for ages ≥65 versus <40 years (P = 0.001).
Conclusions: Adults with diabetes experience glycemic benefit after remote CGM use training, but training time for those >65 years is longer compared with younger adults. Addressing individual training-related needs, including needs that may vary by age, should be considered.
Keywords: older adults; telehealth; telemedicine; type 1 diabetes.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RSW reported personal fees from The Leona M. and Harry B. Helmsley Charitable Trust and Jaeb Center for Health Research during the conduct of the study; grants for participation in multicenter clinical trial through her institution from Eli Lilly, Novo Nordisk, Insulet, Tandem, Amgen, and MannKind outside the submitted work; and discounted devices for clinical research from Dexcom outside the submitted work. RLG reported grants from The Leona M. and Harry B. Helmsley Charitable Trust during the conduct of the study. RMB reported grants from Abbott Diabetes Care, Eli Lilly, Hygieia, Dexcom, Sanofi, Tandem Diabetes Care, Insulet, and Medtronic and consulting and/or advisory board fees paid to his institution from Abbott Diabetes Care, Dexcom, Onduo, Sanofi, Roche, Embecta, and Medtronic during the conduct of the study. CK reported grants from The Leona M. and Harry B. Helmsley Charitable Trust and nonfinancial support from Dexcom during the conduct of the study; grants from JDRF, Diasome, and MannKind outside the submitted work; and nonfinancial support from Tandem, Dexcom, and Insulet outside the submitted work. DK reported grants from Abbott and advisor and/or speaking fees from Abbott, Dexcom, and Jaeb Center for Health Research outside the submitted work. MLJ reported grants from Jaeb Center for Health Research paid to HealthPartners Institute during the conduct of the study and grants from Dexcom given directly to HealthPartners Institute outside the submitted work. BAO reported personal fees from Lagoon Health during the conduct of the study and stock ownership in Abbott Laboratories. SMO reported grants from The Leona M. and Harry B. Helmsley Charitable Trust during the conduct of the study and consulting fees from Cecelia Health and advisory board fees from Dexcom outside the submitted work. TKO reported grants from The Leona M. and Harry B. Helmsley Charitable Trust during the conduct of the study and consulting fees from Cecelia Health, advisory board fees from Dexcom, and an investigator-initiated grant from Abbott Diabetes Care outside the submitted work. RWB reported grants from The Leona M. and Harry B. Helmsley Charitable Trust during the conduct of the study and grants from Insulet, Tandem Diabetes Care, Beta Bionics, Dexcom, and Bigfoot Biomedical; nonfinancial support from Insulet, Tandem Diabetes Care, Beta Bionics, Dexcom, Medtronic, Ascenia, Roche, Eli Lilly, and Novo Nordisk; and consulting fees paid to his institution from Insulet, Tandem Diabetes Care, Beta Bionics, Eli Lilly, Novo Nordisk, Embecta, Hagar, and Ypsomed outside the submitted work. KH reported behavioral expertise consulting fees from Cecelia Health during the conduct of the study. GA reported grants from The Leona M. and Harry B. Helmsley Charitable Trust during the conduct of the study and personal fees from Bayer, Dexcom, and Insulet; nonfinancial support from Eli Lilly; and grants from Dexcom, Eli Lilly, Fractyl Health, Emmes, MannKind, Tandem Diabetes Care, and Welldoc outside the submitted work. No other disclosures were reported.
Figures
References
LinkOut - more resources
Full Text Sources